Anti-Inflammatory Peptide 1PLA2 inhibitor CAS# 118850-71-8 |
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 118850-71-8 | SDF | Download SDF |
| PubChem ID | 164083 | Appearance | Powder |
| Formula | C45H82N12O14S2 | M.Wt | 1079.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | antiflamin 1;antiflammin 1;antiflammin P1 | ||
| Solubility | >107.9mg/mL in DMSO or water | ||
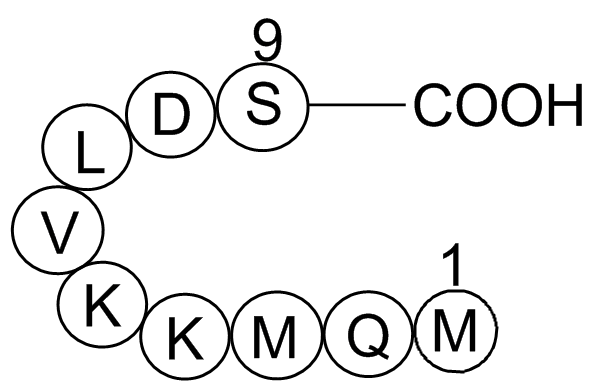
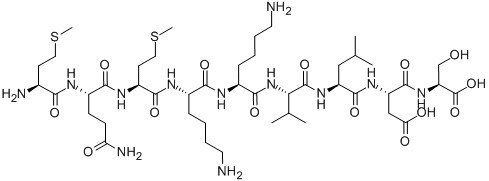
| Sequence | H2N-Met-Gln-Met-Lys-Lys-Val-Leu-Asp-Ser-OH | ||
| Chemical Name | (3S)-3-[[2-[[(2S)-2-[[6-amino-2-[[(2S)-6-amino-2-[[2-[[(2S)-5-amino-2-[[(2S)-2-amino-4-methylsulfanylbutanoyl]amino]-5-oxopentanoyl]amino]-4-methylsulfanylbutanoyl]amino]hexanoyl]amino]hexanoyl]amino]-3-methylbutanoyl]amino]-4-methylpentanoyl]amino]-4-[[(1S)-1-carboxy-2-hydroxyethyl]amino]-4-oxobutanoic acid | ||
| SMILES | CC(C)CC(C(=O)NC(CC(=O)O)C(=O)NC(CO)C(=O)O)NC(=O)C(C(C)C)NC(=O)C(CCCCN)NC(=O)C(CCCCN)NC(=O)C(CCSC)NC(=O)C(CCC(=O)N)NC(=O)C(CCSC)N | ||
| Standard InChIKey | XVZUZGWPOCVGGZ-VYAJKLLMSA-N | ||
| Standard InChI | InChI=1S/C45H82N12O14S2/c1-24(2)21-31(42(67)54-32(22-35(60)61)43(68)56-33(23-58)45(70)71)55-44(69)36(25(3)4)57-41(66)28(12-8-10-18-47)52-38(63)27(11-7-9-17-46)51-40(65)30(16-20-73-6)53-39(64)29(13-14-34(49)59)50-37(62)26(48)15-19-72-5/h24-33,36,58H,7-23,46-48H2,1-6H3,(H2,49,59)(H,50,62)(H,51,65)(H,52,63)(H,53,64)(H,54,67)(H,55,69)(H,56,68)(H,57,66)(H,60,61)(H,70,71)/t26-,27-,28?,29-,30?,31?,32-,33-,36-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Anti-Inflammatory Peptide 1 Dilution Calculator

Anti-Inflammatory Peptide 1 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Anti-Inflammatory Peptide 1 (C45H82N12O14S2), with the sequence H-Met-Gln-Met-Lys-Lys-Val-Leu-Asp-Ser-OH, belongs to the group of synthetic oligopeptides corresponding to a region of high amino-acid sequence similarity between uteroglobin and lipocortin I. The name 'antiflammins' is proposed for those peptides, which are valuable models for the development of novel anti-inflammatory agents of therapeutic importance. Anti-inflammatory peptide 1 possesses a potent anti-inflammatory activity in vivo and is a strong inhibitor of phospholipase A2 (PLA2), whose increased presence and activity results in inflammation and pain at certain bodily sites.
Ref:
1. Argiolas A, Pisano JJ (November 1983). "Facilitation of phospholipase A2 activity by mastoparans, a new class of mast cell degranulating peptides from wasp venom". J. Biol. Chem. 258 (22): 13697–702.
- Ustusolate C
Catalog No.:BCN6755
CAS No.:1188398-15-3
- Ustusol C
Catalog No.:BCN6757
CAS No.:1188398-13-1
- 15,16-Di-O-acetyldarutoside
Catalog No.:BCN6071
CAS No.:1188282-02-1
- 16-O-Acetyldarutigenol
Catalog No.:BCN6070
CAS No.:1188282-01-0
- 9-Hydroxydarutigenol
Catalog No.:BCN6069
CAS No.:1188282-00-9
- 7-Hydroxydarutigenol
Catalog No.:BCN6068
CAS No.:1188281-99-3
- ent-14,16-Epoxy-8-pimarene-3,15-diol
Catalog No.:BCN6067
CAS No.:1188281-98-2
- Ac-Leu-OH
Catalog No.:BCC2967
CAS No.:1188-21-2
- Diosbulbin J
Catalog No.:BCN6066
CAS No.:1187951-06-9
- Diosbulbin I
Catalog No.:BCN6065
CAS No.:1187951-05-8
- Carabrolactone B
Catalog No.:BCN6064
CAS No.:1187925-31-0
- Carabrolactone A
Catalog No.:BCN6063
CAS No.:1187925-30-9
- NBQX
Catalog No.:BCC6624
CAS No.:118876-58-7
- ABT 702 dihydrochloride
Catalog No.:BCC5905
CAS No.:1188890-28-9
- KN-93 Phosphate
Catalog No.:BCC5638
CAS No.:1188890-41-6
- CEP-32496
Catalog No.:BCC1079
CAS No.:1188910-76-0
- Alstoyunine E
Catalog No.:BCN4782
CAS No.:1188932-15-1
- Fmoc-D-Allo-Ile-OH
Catalog No.:BCC3508
CAS No.:118904-37-3
- Lettowienolide
Catalog No.:BCN8038
CAS No.:1189105-39-2
- Denudaquinol
Catalog No.:BCN8035
CAS No.:1189105-40-5
- Balanophonin
Catalog No.:BCN6072
CAS No.:118916-57-7
- 5-(3-Hydroxypropyl)-7-methoxybenzofuran
Catalog No.:BCN1606
CAS No.:118930-92-0
- 1,3-Dihydroxy-4-methoxy-10-methylacridin-9(10H)-one
Catalog No.:BCN1605
CAS No.:1189362-86-4
- (S,S)-2,6-Bis(4-isopropyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8402
CAS No.:118949-61-4
AP-1-Targeted Anti-Inflammatory Activities of the Nanostructured, Self-Assembling S5 Peptide.[Pubmed:26074678]
Mediators Inflamm. 2015;2015:451957.
Peptide-based therapeutics have received increasing attention in medical research. However, the local delivery of such therapeutics poses unique challenges. Self-assembling peptides that use decorated nanofibers are one approach by which these therapeutics may be delivered. We previously found that the self-assembling K5 peptide affects the anti-inflammatory response. The aim of the present study was to investigate another self-assembling peptide, S5. Unlike the K5 peptide which has a positive charge, the S5 peptide has a free hydroxyl (-OH) group. We first examined whether the S5 peptide regulates the inflammatory response in primary cells and found that the S5 peptide reduced the production of prostaglandin E2 (PGE2) and tumor necrosis factor (TNF)-alpha in lipopolysaccharide- (LPS-) treated bone marrow-derived macrophages. Moreover, the S5 peptide significantly downregulated cyclooxygenase- (COX-) 2, TNF-alpha, and interleukin- (IL-) 1beta expression by blocking the nuclear translocation of c-Jun. Consistent with this finding, the S5 peptide diminished the activation of inflammatory signaling enzymes related to p38. The S5 peptide also inhibited the formation of the p38/c-Jun signaling complex in RAW264.7 cells. Similarly, p38 and MKK3/6 were inhibited by the S5 peptide in LPS-activated peritoneal macrophages. Taken together, these results strongly suggest that the S5 peptide could exert anti-inflammatory effects by inhibiting the c-Jun/p38 signaling pathway.
Glucagon-like peptide-1 and vitamin D: anti-inflammatory response in diabetic kidney disease in db/db mice and in cultured endothelial cells.[Pubmed:26991522]
Diabetes Metab Res Rev. 2016 Nov;32(8):805-815.
BACKGROUND: Glucagon-like peptide-1 (GLP-1) is a gut incretin hormone that stimulates insulin secretion and may affect the inflammatory pathways involved in diabetes mellitus. Calcitriol, an active form of vitamin D, plays an important role in renal, endothelial and cardiovascular protection. We evaluated the anti-inflammatory and histologic effects of a GLP-1 analogue (liraglutide) and of calcitriol in a db/db mouse diabetes model and in endothelial cells exposed to a diabetes-like environment. METHODS: Diabetic db/db mice were treated with liraglutide and calcitriol for 14 weeks, after which the kidneys were perfused and removed for mRNA and protein analysis and histology. Endothelial cells were stimulated with advanced glycation end products (AGEs), glucose, liraglutide and calcitriol. Total RNA and protein were extracted and analysed for the expression of selected inflammatory markers. RESULTS: Typical histological changes, glomerular enlargement and mesangial expansion were seen in db/db mice compared with control mice. Glomerular hypertrophy was ameliorated with liraglutide, compared with db/db controls. Liraglutide up-regulated endothelial nitric oxide synthase protein expression compared with the db/db control group and down-regulated p65 protein expression. Calcitriol did not further improve the beneficial effect observed on protein expression. In endothelial cells, liraglutide treatment exhibited a dose-dependent ability to prevent an inflammatory response in the selected markers: thioredoxin-interacting protein, p65, IL6 and IL8. In most gene and protein expressions, addition of calcitriol did not enhance the effect of liraglutide. CONCLUSIONS: The GLP-1 analogue liraglutide prevented the inflammatory response observed in endothelial cells exposed to a diabetes-like environment and in db/db mice at the level of protein expression and significantly ameliorated the glomerular hypertrophy seen in the diabetic control group. Copyright (c) 2016 John Wiley & Sons, Ltd.
Anti-atherogenic and anti-inflammatory properties of glucagon-like peptide-1, glucose-dependent insulinotropic polypepide, and dipeptidyl peptidase-4 inhibitors in experimental animals.[Pubmed:27186361]
J Diabetes Investig. 2016 Apr;7 Suppl 1:80-6.
We reported that native incretins, liraglutide and dipeptidyl peptidase-4 inhibitors (DPP-4i) all confer an anti-atherosclerotic effect in apolipoprotein E-null (Apoe (-/-)) mice. We confirmed the anti-atherogenic property of incretin-related agents in the mouse wire injury model, in which the neointimal formation in the femoral artery is remarkably suppressed. Furthermore, we showed that DPP-4i substantially suppresses plaque formation in coronary arteries with a marked reduction in the accumulation of macrophages in cholesterol-fed rabbits. DPP-4i showed an anti-atherosclerotic effect in Apoe (-/-) mice mainly through the actions of glucagon-like peptide-1 and glucose-dependent insulinotropic polypepide. However, the dual incretin receptor antagonists partially attenuated the suppressive effect of DPP-4i on atherosclerosis in diabetic Apoe (-/-) mice, suggesting an incretin-independent mechanism. Exendin-4 and glucose-dependent insulinotropic polypepide elicited cyclic adenosine monophosphate generation, and suppressed the lipopolysaccharide-induced gene expression of inflammatory molecules, such as interleukin-1beta, interleukin-6 and tumor necrosis factor-alpha, in U937 human monocytes. This suppressive effect, however, was attenuated by an inhibitor of adenylate cyclase and mimicked by 8-bromo-cyclic adenosine monophosphate or forskolin. DPP-4i substantially suppressed the lipopolysaccharide-induced expression of inflammatory cytokines without affecting cyclic adenosine monophosphate generation or cell proliferation. DPP-4i more strongly suppressed the lipopolysaccharide-induced gene expression of inflammatory molecules than incretins, most likely through inactivation of CD26. Glucagon-like peptide-1 and glucose-dependent insulinotropic polypepide suppressed oxidized low-density lipoprotein-induced macrophage foam cell formation in a receptor-dependent manner, which was associated with the downregulation of acyl-coenzyme A cholesterol acyltransferase-1 and CD36, as well as the up-regulation of adenosine triphosphate-binding cassette transporter A1. Our studies strongly suggest that incretin-related agents have favorable effects on macrophage-driven atherosclerosis in experimental animals.
Sulforaphane exerts its anti-inflammatory effect against amyloid-beta peptide via STAT-1 dephosphorylation and activation of Nrf2/HO-1 cascade in human THP-1 macrophages.[Pubmed:26827637]
Neurobiol Aging. 2016 Feb;38:1-10.
Alzheimer's disease (AD) is the most common neurodegenerative disorder worldwide, accounting for most cases of dementia in elderly individuals, and effective therapies are still lacking. This study was designed to investigate the anti-inflammatory properties of sulforaphane against Abeta1-42 monomers in human THP-1 microglia-like cells. The results showed that sulforaphane preferentially inhibited cathepsin B- and caspase-1-dependent NLRP3 inflammasome activation induced by mostly Abeta1-42 monomers, an effect that potently reduced excessive secretion of the proinflammatory cytokine interleukin-1beta (IL-1beta). Subsequent mechanistic studies revealed that sulforaphane mitigated the activation of signal transducer and activator of transcription-1 induced by Abeta1-42 monomers. Sulforaphane also increased nuclear factor erythroid 2-related factor 2 (Nrf2) nuclear translocation, which was followed by upregulation of heme-oxygenase 1 (HO-1). The anti-inflammatory effect of sulforaphane on Abeta1-42-induced IL-1beta production was diminished by small interfering RNA-mediated knockdown of Nrf2 or HO-1. Moreover, sulforaphane significantly attenuated the levels of microRNA-146a, which is selectively upregulated in the temporal cortex and hippocampus of AD brains. The aforementioned effects of sulforaphane were replicated by the tyrosine kinase inhibitor, herbimycin A, and Nrf2 activator. These results indicate that signal transducer and activator of transcription-1 dephosphorylation, HO-1 and its upstream effector, Nrf2, play a pivotal role in triggering an anti-inflammatory signaling cascade of sulforaphane that results in decreases of IL-1beta release and microRNA-146a production in Abeta1-42-stimulated human microglia-like cells. These findings suggest that the phytochemical sulforaphane has a potential application in AD therapeutics.


