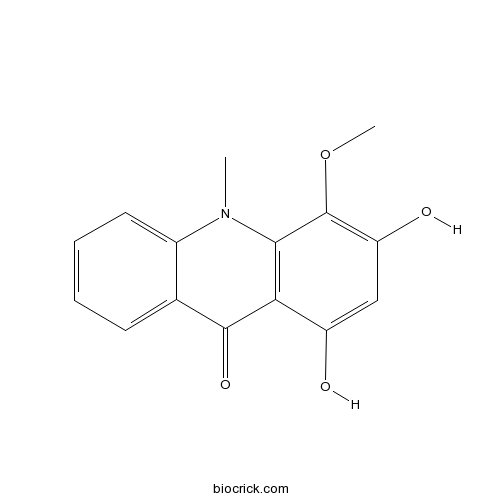
1,3-Dihydroxy-4-methoxy-10-methylacridin-9(10H)-oneCAS# 1189362-86-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1189362-86-4 | SDF | Download SDF |
| PubChem ID | 44179836 | Appearance | Yellow solid |
| Formula | C15H13NO4 | M.Wt | 271.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,3-dihydroxy-4-methoxy-10-methylacridin-9-one | ||
| SMILES | CN1C2=CC=CC=C2C(=O)C3=C1C(=C(C=C3O)O)OC | ||
| Standard InChIKey | YIBYVKSDVRSLGT-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

1,3-Dihydroxy-4-methoxy-10-methylacridin-9(10H)-one Dilution Calculator

1,3-Dihydroxy-4-methoxy-10-methylacridin-9(10H)-one Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.686 mL | 18.4298 mL | 36.8596 mL | 73.7191 mL | 92.1489 mL |
| 5 mM | 0.7372 mL | 3.686 mL | 7.3719 mL | 14.7438 mL | 18.4298 mL |
| 10 mM | 0.3686 mL | 1.843 mL | 3.686 mL | 7.3719 mL | 9.2149 mL |
| 50 mM | 0.0737 mL | 0.3686 mL | 0.7372 mL | 1.4744 mL | 1.843 mL |
| 100 mM | 0.0369 mL | 0.1843 mL | 0.3686 mL | 0.7372 mL | 0.9215 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5-(3-Hydroxypropyl)-7-methoxybenzofuran
Catalog No.:BCN1606
CAS No.:118930-92-0
- Balanophonin
Catalog No.:BCN6072
CAS No.:118916-57-7
- Denudaquinol
Catalog No.:BCN8035
CAS No.:1189105-40-5
- Lettowienolide
Catalog No.:BCN8038
CAS No.:1189105-39-2
- Fmoc-D-Allo-Ile-OH
Catalog No.:BCC3508
CAS No.:118904-37-3
- Alstoyunine E
Catalog No.:BCN4782
CAS No.:1188932-15-1
- CEP-32496
Catalog No.:BCC1079
CAS No.:1188910-76-0
- KN-93 Phosphate
Catalog No.:BCC5638
CAS No.:1188890-41-6
- ABT 702 dihydrochloride
Catalog No.:BCC5905
CAS No.:1188890-28-9
- NBQX
Catalog No.:BCC6624
CAS No.:118876-58-7
- Anti-Inflammatory Peptide 1
Catalog No.:BCC1006
CAS No.:118850-71-8
- Ustusolate C
Catalog No.:BCN6755
CAS No.:1188398-15-3
- (S,S)-2,6-Bis(4-isopropyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8402
CAS No.:118949-61-4
- Ethyl ganoderate J
Catalog No.:BCN3486
CAS No.:1189555-95-0
- Mephedrone hydrochloride
Catalog No.:BCC6183
CAS No.:1189726-22-4
- Fumitremorgin C
Catalog No.:BCC7507
CAS No.:118974-02-0
- 1-O-Deacetyl-2alpha-hydroxykhayanolide E
Catalog No.:BCN1604
CAS No.:1189801-51-1
- Viscumneoside III
Catalog No.:BCN7698
CAS No.:118985-27-6
- 8-Amino-2-naphthalenesulfonic acid
Catalog No.:BCC8783
CAS No.:119-28-8
- Methyl salicylate
Catalog No.:BCN5372
CAS No.:119-36-8
- 7-Anilino-4-hydroxy-2-naphthalenesulfonic acid
Catalog No.:BCC8777
CAS No.:119-40-4
- p-Anisoin
Catalog No.:BCC9113
CAS No.:119-52-8
- Benzoin
Catalog No.:BCC8854
CAS No.:119-53-9
- Benzophenone
Catalog No.:BCC8859
CAS No.:119-61-9
Vibrational spectroscopy investigation using M06-2X and B3LYP methods analysis on the structure of 2-Trifluoromethyl-10H-benzo[4,5]-imidazo[1,2-a]pyrimidin-4-one.[Pubmed:24662759]
Spectrochim Acta A Mol Biomol Spectrosc. 2014 Jul 15;128:109-18.
In this study, the experimental and theoretical vibrational frequencies of a newly synthesized bioactive agent namely, 2-Trifluoromethyl-10H-benzo[4,5]-imidazo[1,2-a]pyrimidin-4-one (TIP) have been investigated. The experimental FT-IR (4000-400 cm(-1)) and Laser-Raman spectra (4000-100 cm(-1)) of the molecule in solid phase have been recorded. The theoretical vibrational frequencies and the optimized geometric parameters (bond lengths and bond angles) have been calculated using density functional theory (DFT/B3LYP: Becke, 3-parameter, Lee-Yang-Parr) and M06-2X (the highly parametrized, empirical exchange correlation function) quantum chemical methods with 6-311++G(d,p) basis set by Gaussian 09W software, for the first time. The assignments of the vibrational frequencies have been done by potential energy distribution (PED) analysis using VEDA 4 software. The theoretical optimized geometric parameters and vibrational frequencies have been found to be in good agreement with the corresponding experimental data and results in the literature. In addition, the highest occupied molecular orbital (HOMO) energy, the lowest unoccupied molecular orbital (LUMO) energy and the other related molecular energy values of the compound have been investigated using the same theoretical calculations.
Crystal structure of (Z)-1-(ferrocenylethyn-yl)-10-(phenyl-imino)-anthracen-9(10H)-one from synchrotron X-ray powder diffraction.[Pubmed:25552995]
Acta Crystallogr Sect E Struct Rep Online. 2014 Nov 26;70(Pt 12):573-6.
In the title compound, [Fe(C5H5)(C27H16NO)], designed and synthesized to explore a new electron-donor (D) and -acceptor (A) conjugated complex, the two cyclo-penta-dienyl rings adopt an eclipsed conformation. The anthracene tricycle is distorted towards a butterfly conformation and the mean planes of the outer benzene rings are inclined each to other at 22.7 (3) degrees . In the crystal, mol-ecules are paired into inversion dimers via pi-pi inter-actions. Weak inter-molecular C-Hcdots, three dots, centeredpi inter-actions link further these dimers into one-dimensional columns along the b axis, with the ferrocenylethynyl arms arranged between the stacks to fill the voids.
1,8-Naphthyridines IX. Potent anti-inflammatory and/or analgesic activity of a new group of substituted 5-amino[1,2,4]triazolo[4,3-a][1,8]naphthyridine-6-carboxamides, of some their Mannich base derivatives and of one novel substituted 5-amino-10-oxo-10H-pyrimido[1,2-a][1,8]naphthyridine-6-carboxamide derivative.[Pubmed:25194932]
Eur J Med Chem. 2014 Oct 30;86:394-405.
A new group of 5-(alkylamino)-9-isopropyl[1,2,4]triazolo[4,3-a][1,8]naphthyridine derivatives bearing a CONHR group at the 6-position (1c-g), designed to obtain new effective analgesic and/or anti-inflammatory agents, were synthesized and tested along with three new 9-alkyl-5-(4-alkyl-1-piperazinyl)-N,N-diethyl [1,2,4]triazolo[4,3-a][1,8]naphthyridine-6-carboxamides (2b-d). Besides, a new class of analogues of compounds 1 and 2, bearing a Mannich base moiety at the 9-position (12a-d), as well as the novel N,N-diethyl-5-(isobutylamino)-8-methyl-10-oxo-10H-pyrimido[1,2-a][1,8]naphthyridi ne-6-carboxamide (15) were prepared and tested. Compounds 1c-g exhibited very interesting anti-inflammatory properties in rats, whereas compounds 2b-d and 15 proved to be endowed with prevalent analgesic activity frequently associated with sedative effects in mice. On the contrary, the Mannich bases 12a-d resulted inactive. The most effective (80% inhibition of oedema) and potent (threshold dose 1.6 mg kg(-1) with 31% inhibition of oedema) anti-inflammatory compound 1d did not show gastrolesive effects following 100 mg kg(-1) oral administration in rats.
Theoretical analysis of the mechanism and regioselectivity of the 1, 3-dipolar cycloaddition of E-3-(dimethylamino)-1-(10H-phenothiazin-2-yl)prop-2-en-1-one with some nitrilimines using DFT and the distortion/interaction model.[Pubmed:26085424]
Acta Chim Slov. 2015;62(2):403-10.
The regiochemistry of 1,3-dipolar cycloaddition reactions of E-3-(dimethylamino)-1-(10H-phenothiazin-2-yl) prop-2-en-1-one with some nitrilimines were investigated using density functional theory (DFT) -based reactivity indexes, activation energy calculations and the distortion/interaction model at B3LYP/6-311G(d,p) level of theory. Analysis of the geometries and bond orders (BOs) at the TS structures associated with the different reaction pathways shows that these 1,3-dipolar cycloaddition reactions occur via an asynchronous concerted mechanism.


