2-CarboxybenzaldehydeCAS# 119-67-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 119-67-5 | SDF | Download SDF |
| PubChem ID | 8406 | Appearance | Cryst. |
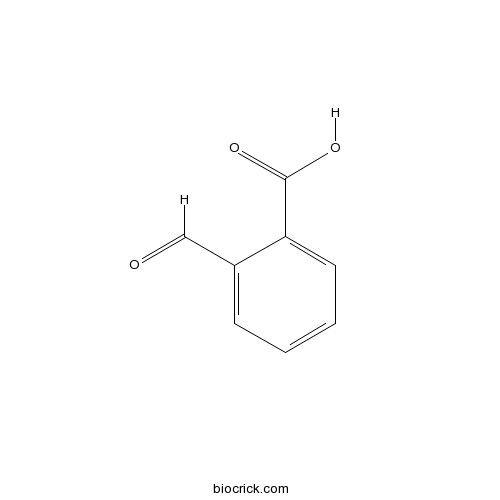
| Formula | C8H6O3 | M.Wt | 150.13 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-formylbenzoic acid | ||
| SMILES | C1=CC=C(C(=C1)C=O)C(=O)O | ||
| Standard InChIKey | DYNFCHNNOHNJFG-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | The major peak of 2-Carboxybenzaldehyde reductase activity in human liver co-purifies with hAFAR protein. Sulphydryl substances and some proteins play important roles in preserving the molecular and catalytic properties of 2-Carboxybenzaldehyde reductase. |
| Targets | NADPH-oxidase |
| In vitro | Purification and molecular properties of 2-carboxybenzaldehyde (CBA) reductase from phenobarbital-treated rat liver.[Pubmed: 1441592]Xenobiotica. 1992 Jun;22(6):691-9.1. A rat liver cytosol enzyme, tentatively named CBA reductase, catalyses the conversion of 2-Carboxybenzaldehyde (CBA) to 2-hydroxymethyl benzoic acid in the presence of NADH (or NADPH). CBA reductase is useful for exploring the mechanism of in vitro enzyme induction, as the enzyme can be induced by phenobarbital (PB) both in vivo and in vitro. Induction of 2-carboxybenzaldehyde reductase by phenobarbital in primary culture of rat hepatocytes.[Pubmed: 3541933]Biochem Biophys Res Commun. 1986 Dec 15;141(2):488-93.
|
| Kinase Assay | Effect of L-methionine on 2-carboxybenzaldehyde reductase induction by phenobarbital in primary cultures of rat hepatocytes.[Pubmed: 1991334]Chem Biol Interact. 1991;77(2):149-58.Effects of phenobarbital (PB) and L-methionine on 2-Carboxybenzaldehyde (CBA) reductase in rat hepatocyte primary culture were examined. |
| Structure Identification | Spectrochim Acta A Mol Biomol Spectrosc. 2014 Aug 14;129:333-8.Synthesis, spectroscopic, anticancer and antibacterial studies of Ni(II) and Cu(II) complexes with 2-carboxybenzaldehyde thiosemicarbazone.[Pubmed: 24747857]Ni(II) and Cu(II) complexes of 2-Carboxybenzaldehyde thiosemicarbazone (L) were synthesized and investigated by their spectral and analytical data. These newly synthesized complexes have a composition of M(L)X(H2O)2 (where M=Ni(II), Cu(II) and X=Cl(-), NO3(-), CH3COO(-)) and (L) is the tridentate Schiff base ligand. The ligand and its complexes have been characterized on the basis of analytical, molar conductivity, magnetic susceptibility measurements, FT-IR, ESR, (1)H NMR and electronic spectral analysis. Biochem J. 1998 May 15;332 ( Pt 1):21-34.Molecular cloning, expression and catalytic activity of a human AKR7 member of the aldo-keto reductase superfamily: evidence that the major 2-carboxybenzaldehyde reductase from human liver is a homologue of rat aflatoxin B1-aldehyde reductase.[Pubmed: 9576847]The masking of charged amino or carboxy groups by N-phthalidylation and O-phthalidylation has been used to improve the absorption of many drugs, including ampicillin and 5-fluorouracil. Following absorption of such prodrugs, the phthalidyl group is hydrolysed to release 2-Carboxybenzaldehyde (2-CBA) and the pharmaceutically active compound; in humans, 2-Carboxybenzaldehyde is further metabolized to 2-hydroxymethylbenzoic acid by reduction of the aldehyde group. |

2-Carboxybenzaldehyde Dilution Calculator

2-Carboxybenzaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.6609 mL | 33.3045 mL | 66.6089 mL | 133.2179 mL | 166.5223 mL |
| 5 mM | 1.3322 mL | 6.6609 mL | 13.3218 mL | 26.6436 mL | 33.3045 mL |
| 10 mM | 0.6661 mL | 3.3304 mL | 6.6609 mL | 13.3218 mL | 16.6522 mL |
| 50 mM | 0.1332 mL | 0.6661 mL | 1.3322 mL | 2.6644 mL | 3.3304 mL |
| 100 mM | 0.0666 mL | 0.333 mL | 0.6661 mL | 1.3322 mL | 1.6652 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Benzophenone
Catalog No.:BCC8859
CAS No.:119-61-9
- Benzoin
Catalog No.:BCC8854
CAS No.:119-53-9
- p-Anisoin
Catalog No.:BCC9113
CAS No.:119-52-8
- 7-Anilino-4-hydroxy-2-naphthalenesulfonic acid
Catalog No.:BCC8777
CAS No.:119-40-4
- Methyl salicylate
Catalog No.:BCN5372
CAS No.:119-36-8
- 8-Amino-2-naphthalenesulfonic acid
Catalog No.:BCC8783
CAS No.:119-28-8
- Viscumneoside III
Catalog No.:BCN7698
CAS No.:118985-27-6
- 1-O-Deacetyl-2alpha-hydroxykhayanolide E
Catalog No.:BCN1604
CAS No.:1189801-51-1
- Fumitremorgin C
Catalog No.:BCC7507
CAS No.:118974-02-0
- Mephedrone hydrochloride
Catalog No.:BCC6183
CAS No.:1189726-22-4
- Ethyl ganoderate J
Catalog No.:BCN3486
CAS No.:1189555-95-0
- (S,S)-2,6-Bis(4-isopropyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8402
CAS No.:118949-61-4
- 5-Amino-2-naphthalenesulfonic acid
Catalog No.:BCC8732
CAS No.:119-79-9
- 3,4-Dihydrocoumarin
Catalog No.:BCN6793
CAS No.:119-84-6
- 2,2'-Biquinoline
Catalog No.:BCC8489
CAS No.:119-91-5
- Abiesinol F
Catalog No.:BCN6418
CAS No.:1190070-91-7
- 4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide
Catalog No.:BCC8672
CAS No.:119018-29-0
- M2 ion channel blocker
Catalog No.:BCC1726
CAS No.:1190215-03-2
- Sarcandrone A
Catalog No.:BCN6073
CAS No.:1190225-47-8
- Sarcandrone B
Catalog No.:BCN6074
CAS No.:1190225-48-9
- PSI-7977
Catalog No.:BCC1871
CAS No.:1190307-88-0
- PSI-7976
Catalog No.:BCC5138
CAS No.:1190308-01-0
- EB 47
Catalog No.:BCC2452
CAS No.:1190332-25-2
- MRT67307
Catalog No.:BCC1779
CAS No.:1190378-57-4
Molecular cloning, expression and catalytic activity of a human AKR7 member of the aldo-keto reductase superfamily: evidence that the major 2-carboxybenzaldehyde reductase from human liver is a homologue of rat aflatoxin B1-aldehyde reductase.[Pubmed:9576847]
Biochem J. 1998 May 15;332 ( Pt 1):21-34.
The masking of charged amino or carboxy groups by N-phthalidylation and O-phthalidylation has been used to improve the absorption of many drugs, including ampicillin and 5-fluorouracil. Following absorption of such prodrugs, the phthalidyl group is hydrolysed to release 2-Carboxybenzaldehyde (2-CBA) and the pharmaceutically active compound; in humans, 2-CBA is further metabolized to 2-hydroxymethylbenzoic acid by reduction of the aldehyde group. In the present work, the enzyme responsible for the reduction of 2-CBA in humans is identified as a homologue of rat aflatoxin B1-aldehyde reductase (rAFAR). This novel human aldo-keto reductase (AKR) has been cloned from a liver cDNA library, and together with the rat protein, establishes the AKR7 family of the AKR superfamily. Unlike its rat homologue, human AFAR (hAFAR) appears to be constitutively expressed in human liver, and is widely expressed in extrahepatic tissues. The deduced human and rat protein sequences share 78% identity and 87% similarity. Although the two AKR7 proteins are predicted to possess distinct secondary structural features which distinguish them from the prototypic AKR1 family of AKRs, the catalytic- and NADPH-binding residues appear to be conserved in both families. Certain of the predicted structural features of the AKR7 family members are shared with the AKR6 beta-subunits of voltage-gated K+-channels. In addition to reducing the dialdehydic form of aflatoxin B1-8,9-dihydrodiol, hAFAR shows high affinity for the gamma-aminobutyric acid metabolite succinic semialdehyde (SSA) which is structurally related to 2-CBA, suggesting that hAFAR could function as both a SSA reductase and a 2-CBA reductase in vivo. This hypothesis is supported in part by the finding that the major peak of 2-CBA reductase activity in human liver co-purifies with hAFAR protein.
Effect of L-methionine on 2-carboxybenzaldehyde reductase induction by phenobarbital in primary cultures of rat hepatocytes.[Pubmed:1991334]
Chem Biol Interact. 1991;77(2):149-58.
Effects of phenobarbital (PB) and L-methionine on 2-Carboxybenzaldehyde (CBA) reductase in rat hepatocyte primary culture were examined. Inclusion of PB in the culture medium markedly enhanced the CBA reductase activity while L-methionine, which elevates the cellular glutathione (GSH) level, suppressed the stimulatory effect of PB. This suppression, though less pronounced, was also found with other precursors of GSH biosynthesis. GSH-depletors, buthionine sulfoximine (BSO) or diethylmaleate (DEM), enhanced the CBA reductase activity suggesting that GSH plays an important role in enzyme induction.
Synthesis, spectroscopic, anticancer and antibacterial studies of Ni(II) and Cu(II) complexes with 2-carboxybenzaldehyde thiosemicarbazone.[Pubmed:24747857]
Spectrochim Acta A Mol Biomol Spectrosc. 2014 Aug 14;129:333-8.
Ni(II) and Cu(II) complexes of 2-Carboxybenzaldehyde thiosemicarbazone (L) were synthesized and investigated by their spectral and analytical data. These newly synthesized complexes have a composition of M(L)X(H2O)2 (where M=Ni(II), Cu(II) and X=Cl(-), NO3(-), CH3COO(-)) and (L) is the tridentate Schiff base ligand. The ligand and its complexes have been characterized on the basis of analytical, molar conductivity, magnetic susceptibility measurements, FT-IR, ESR, (1)H NMR and electronic spectral analysis. All the compounds were non-electrolytic in nature. On the basis of spectral studies an octahedral geometry has been assigned for Ni(II) and a tetragonal geometry for Cu(II) complexes. The ligand and its metal complexes were screened for their anticancer studies against human breast cancer cell lines MCF-7 and calculated minimum inhibitory concentration and also for antibacterial activity using Kirby-Bauer single disk susceptibility test.
Induction of 2-carboxybenzaldehyde reductase by phenobarbital in primary culture of rat hepatocytes.[Pubmed:3541933]
Biochem Biophys Res Commun. 1986 Dec 15;141(2):488-93.
When rats were treated with phenobarbital (PB), the activity of CBA reductase, which catalyzes the conversion of 2-Carboxybenzaldehyde (CBA) to 2-hydroxymethylbenzoic acid (HMB), in the liver was markedly enhanced. Likewise, addition of PB to the primary culture of rat hepatocytes increased the activity of CBA reductase. The enzyme recovered from cell lysate of cultured cells showed the same characteristics in molecular and catalytic properties as the enzyme purified from the livers of the rats treated with PB. Experiments with cycloheximide suggest that de novo synthesis of the enzyme protein is enhanced by PB in primary culture.
Purification and molecular properties of 2-carboxybenzaldehyde (CBA) reductase from phenobarbital-treated rat liver.[Pubmed:1441592]
Xenobiotica. 1992 Jun;22(6):691-9.
1. A rat liver cytosol enzyme, tentatively named CBA reductase, catalyses the conversion of 2-Carboxybenzaldehyde (CBA) to 2-hydroxymethyl benzoic acid in the presence of NADH (or NADPH). CBA reductase is useful for exploring the mechanism of in vitro enzyme induction, as the enzyme can be induced by phenobarbital (PB) both in vivo and in vitro. 2. Possible involvement of glutathione (GSH) in gene expression was suggested by a recent study with cultured rat hepatocytes. 3. CBA reductase was purified about 200-fold by a combination of column chromatography and isoelectric focusing in the presence of mercaptoethanol. 4. The ability to form 2-hydroxymethyl benzoic acid was lost when the enzyme was chromatographed on a hydroxylapatite column in the absence of mercaptoethanol; however, it was restored if sulphydryl compounds or bovine serum albumin was added to the eluate from the column. 5. Gel filtration showed the molecular sizes of CBA reductase from the 105,000g supernatant fraction of rat liver extracts and the purified preparation were 64 kDa and 49 kDa, respectively. 6. The results suggest that sulphydryl substances and some proteins play important roles in preserving the molecular and catalytic properties of CBA reductase.


