PSI-7977Antiviral agents for chronic HCV infection CAS# 1190307-88-0 |
- Tegobuvir
Catalog No.:BCC1991
CAS No.:1000787-75-6
- VX-222 (VCH-222, Lomibuvir)
Catalog No.:BCC2108
CAS No.:1026785-59-0
- PSI-7976
Catalog No.:BCC5138
CAS No.:1190308-01-0
- R-1479
Catalog No.:BCC1878
CAS No.:478182-28-4
- PSI-6130
Catalog No.:BCC1870
CAS No.:817204-33-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1190307-88-0 | SDF | Download SDF |
| PubChem ID | 45375808 | Appearance | Powder |
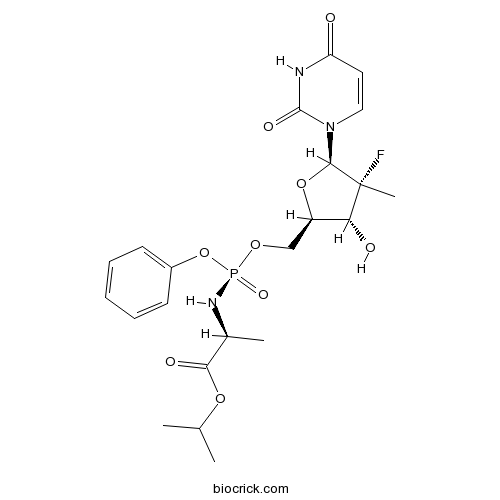
| Formula | C22H29FN3O9P | M.Wt | 529.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GS 7977; sofosbuvir | ||
| Solubility | DMSO : 100 mg/mL (188.88 mM; Need ultrasonic) H2O : 25 mg/mL (47.22 mM; ultrasonic and warming and heat to 50°C) | ||
| Chemical Name | propan-2-yl (2S)-2-[[[(2R,3R,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyloxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate | ||
| SMILES | CC(C)OC(=O)C(C)NP(=O)(OCC1C(C(C(O1)N2C=CC(=O)NC2=O)(C)F)O)OC3=CC=CC=C3 | ||
| Standard InChIKey | TTZHDVOVKQGIBA-IQWMDFIBSA-N | ||
| Standard InChI | InChI=1S/C22H29FN3O9P/c1-13(2)33-19(29)14(3)25-36(31,35-15-8-6-5-7-9-15)32-12-16-18(28)22(4,23)20(34-16)26-11-10-17(27)24-21(26)30/h5-11,13-14,16,18,20,28H,12H2,1-4H3,(H,25,31)(H,24,27,30)/t14-,16+,18+,20+,22+,36-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PSI-7977 is a phosphoramidate prodrug of PSI-7851. | |||||
| Targets | HCV RNA-dependent RNA polymerase | |||||
| IC50 | 92 nM (EC50) | |||||
| Cell experiment: [1] | |
| Cell lines | Non-infected Huh7.5.1 cells and JFH-1-infected Huh7.5.1 cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 24 hours |
| Applications | PSI-7977 prevented PKR activation/ phosphory-lation in infected cells (5-6-fold decrease) and had no effect on the expression of inactive/non-phosphorylated PKR. It had no effect on the IFN-induced expression of non-phosphorylated and phosphorylated STAT1. |
| Animal experiment: [2] | |
| Animal models | TK-NOG mice with non-humanized (control) or humanized livers |
| Dosage form | Oral administration, 44 or 440 mg/kg/d, for 14 days |
| Application | The average plasma ALT levels in mice with humanized livers in the 440- and 44-mg/kg/d treatment groups were below the upper limit of normal, and were not significantly different from those measured in vehicle-treated mice with humanized livers. The plasma lactate levels were also not elevated in or control mice or mice with humanized livers receiving either dose of PSI-7977. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Bobardt M, Chatterji U, Lim P, et al. Both Cyclophilin Inhibitors and Direct-Acting Antivirals Prevent PKR Activation in HCV-Infected Cells. The open virology journal, 2014, 8: 1. [2] Xu D, Nishimura T, Nishimura S, et al. Fialuridine Induces Acute Liver Failure in Chimeric TK-NOG Mice: A Model for Detecting Hepatic Drug Toxicity Prior to Human Testing. PLoS medicine, 2014, 11(4): e1001628. | |

PSI-7977 Dilution Calculator

PSI-7977 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8888 mL | 9.4438 mL | 18.8875 mL | 37.775 mL | 47.2188 mL |
| 5 mM | 0.3778 mL | 1.8888 mL | 3.7775 mL | 7.555 mL | 9.4438 mL |
| 10 mM | 0.1889 mL | 0.9444 mL | 1.8888 mL | 3.7775 mL | 4.7219 mL |
| 50 mM | 0.0378 mL | 0.1889 mL | 0.3778 mL | 0.7555 mL | 0.9444 mL |
| 100 mM | 0.0189 mL | 0.0944 mL | 0.1889 mL | 0.3778 mL | 0.4722 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PSI-7977 is a Nucleotide Inhibitor of Hepatitis C Virus [2]
Hepatitis C virus (HCV) is a disease of liver cirrhosis and development of cancer in liver (hepatocellular carcinoma).
Results from studies using GT 1a (H77)-, 1b (Con1)-, and 2a (JFH-1)-derived replicons and chimeric replicons with the NS5B region fromGT 2a (J6), 2b, and 3a clearly showed that PSI-7977 is a potent HCV inhibitor across NS5B proteins from different isolates. PSI-7977 inhibited the enzymatic activity of NS5B polymerase from GTs 1 to 4 with similar 50% inhibitory concentrations [1]. PSI-7977 is a potent HCV inhibitor with broad genotype coverage. Cross-resistance and selection studies showed that S282T is likely the amino acid change that will be selected by PSI-7977 across various genotypes and subtypes. JFH-1 is a highly unique strain capable of efficient replication and infection, and this particular isolate appeared to require additional amino acid changes together with S282T to reduce the activity of PSI-7977. [3]
References:
[1] Lam AM, Murakami E, Espiritu C et al. PSI-7851, a pronucleotide of beta-D-2'-deoxy-2'-fluoro-2'-C-methyluridine monophosphate, is a potent and pan-genotype inhibitor of hepatitis C virus replication. Antimicrob Agents Chemother. 2010 Aug;54(8):3187-96.
[2] Abdo A. Elfiky, Wael M. Elshemey , Wissam A. Gawad , Omar S. Desoky Molecular Modeling Comparison of the Performance of NS5b Polymerase Inhibitor (PSI-7977) on Prevalent HCV Genotypes. Protein J (2013) 32:75–80.
[3] Angela M. Lam, Christine Espiritu, Shalini Bansal et al. Genotype and Subtype Profiling of PSI-7977 as a Nucleotide Inhibitor of Hepatitis C Virus. Antimicrobial Agents and Chemotherapy June 2012 Volume 56 Number 6 p 3359–3368.
- Sarcandrone B
Catalog No.:BCN6074
CAS No.:1190225-48-9
- Sarcandrone A
Catalog No.:BCN6073
CAS No.:1190225-47-8
- M2 ion channel blocker
Catalog No.:BCC1726
CAS No.:1190215-03-2
- 4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide
Catalog No.:BCC8672
CAS No.:119018-29-0
- Abiesinol F
Catalog No.:BCN6418
CAS No.:1190070-91-7
- 2,2'-Biquinoline
Catalog No.:BCC8489
CAS No.:119-91-5
- 3,4-Dihydrocoumarin
Catalog No.:BCN6793
CAS No.:119-84-6
- 5-Amino-2-naphthalenesulfonic acid
Catalog No.:BCC8732
CAS No.:119-79-9
- 2-Carboxybenzaldehyde
Catalog No.:BCN2274
CAS No.:119-67-5
- Benzophenone
Catalog No.:BCC8859
CAS No.:119-61-9
- Benzoin
Catalog No.:BCC8854
CAS No.:119-53-9
- p-Anisoin
Catalog No.:BCC9113
CAS No.:119-52-8
- PSI-7976
Catalog No.:BCC5138
CAS No.:1190308-01-0
- EB 47
Catalog No.:BCC2452
CAS No.:1190332-25-2
- MRT67307
Catalog No.:BCC1779
CAS No.:1190378-57-4
- Euchrenone B1
Catalog No.:BCN3575
CAS No.:119061-09-5
- Fmoc-Asp-OH
Catalog No.:BCC3085
CAS No.:119062-05-4
- Fluorobexarotene
Catalog No.:BCC6110
CAS No.:1190848-23-7
- Phellolactone
Catalog No.:BCN3467
CAS No.:1190897-23-4
- Linolenic acid ethyl ester
Catalog No.:BCN8333
CAS No.:1191-41-9
- 1-Acetoxy-5-deacetylbaccatin I
Catalog No.:BCN6357
CAS No.:119120-27-3
- IOWH-032
Catalog No.:BCC3922
CAS No.:1191252-49-9
- Ganomycin I
Catalog No.:BCN3504
CAS No.:1191255-15-8
- B-Raf inhibitor 1 dihydrochloride
Catalog No.:BCC4183
CAS No.:1191385-19-9
Discovery of a beta-d-2'-deoxy-2'-alpha-fluoro-2'-beta-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus.[Pubmed:20845908]
J Med Chem. 2010 Oct 14;53(19):7202-18.
Hepatitis C virus (HCV) is a global health problem requiring novel approaches for effective treatment of this disease. The HCV NS5B polymerase has been demonstrated to be a viable target for the development of HCV therapies. beta-d-2'-Deoxy-2'-alpha-fluoro-2'-beta-C-methyl nucleosides are selective inhibitors of the HCV NS5B polymerase and have demonstrated potent activity in the clinic. Phosphoramidate prodrugs of the 5'-phosphate derivative of the beta-d-2'-deoxy-2'-alpha-fluoro-2'-beta-C-methyluridine nucleoside were prepared and showed significant potency in the HCV subgenomic replicon assay (<1 muM) and produced high levels of triphosphate 6 in primary hepatocytes and in the livers of rats, dogs, and monkeys when administered in vivo. The single diastereomer 51 of diastereomeric mixture 14 was crystallized, and an X-ray structure was determined establishing the phosphoramidate stereochemistry as Sp, thus correlating for the first time the stereochemistry of a phosphoramidate prodrug with biological activity. 51 (PSI-7977) was selected as a clinical development candidate.
Synthesis of stable isotope labeled analogs of the anti-hepatitis C virus nucleotide prodrugs PSI-7977 and PSI-352938.[Pubmed:22060553]
Nucleosides Nucleotides Nucleic Acids. 2011 Nov;30(11):886-96.
In order to support bioanalytical LC/MS method development and plasma sample analysis in preclinical and clinical studies of the anti-hepatitis C-virus nucleotides, PSI-7977 and PSI-352938, the corresponding stable isotope labeled forms were prepared. These labeled compounds were prepared by addition reaction of the freshly prepared Grignard reagent (13)CD(3)MgI to the corresponding 2 '-ketone nucleosides followed by fluorination of the resulting carbinol with DAST. As expected, these 2 '-C-(trideuterated-(13)C-methyl) nucleotide prodrugs showed similar anti-HCV activity to that of the corresponding unlabeled ones.
Molecular modeling comparison of the performance of NS5b polymerase inhibitor (PSI-7977) on prevalent HCV genotypes.[Pubmed:23322006]
Protein J. 2013 Jan;32(1):75-80.
The current available treatment for hepatitis C virus (HCV)-the causative of liver cirrhosis and development of liver cancer-is a dual therapy using modified interferon and ribavirin. While this regimen increases the sustained viral response rate up to 40-80 % in different genotypes, unfortunately, it is poorly tolerated by patients. PSI-7977, a prodrug for PSI-7409, is a Non-Structural 5b (NS5b) polymerase nucleoside inhibitor that is currently in phase III clinical trials. The activated PSI-7977 is a direct acting antiviral (DAA) drug that acts on NS5b polymerase of HCV through a coordination bond with the two Mg(+2) present at the GDD active site motif. The present work utilizes a molecular modeling approach for studying the interaction between the activated PSI-7977 and the 12 amino acids constituting a 5 A region surrounding the GDD active triad motif for HCV genotypes 1a, 2b, 3b and 4a. The analysis of the interaction parameters suggests that PSI-7977 is probably a better DAA drug for HCV genotypes 1a and 3b rather than genotypes 2b and 4a.
Genotype and subtype profiling of PSI-7977 as a nucleotide inhibitor of hepatitis C virus.[Pubmed:22430955]
Antimicrob Agents Chemother. 2012 Jun;56(6):3359-68.
PSI-7977, a prodrug of 2'-F-2'-C-methyluridine monophosphate, is the purified diastereoisomer of PSI-7851 and is currently being investigated in phase 3 clinical trials for the treatment of hepatitis C. In this study, we profiled the activity of PSI-7977 and its ability to select for resistance using a number of different replicon cells. Results showed that PSI-7977 was active against genotype (GT) 1a, 1b, and 2a (strain JFH-1) replicons and chimeric replicons containing GT 2a (strain J6), 2b, and 3a NS5B polymerase. Cross-resistance studies using GT 1b replicons confirmed that the S282T change conferred resistance to PSI-7977. Subsequently, we evaluated the ability of PSI-7977 to select for resistance using GT 1a, 1b, and 2a (JFH-1) replicon cells. S282T was the common mutation selected among all three genotypes, but while it conferred resistance to PSI-7977 in GT 1a and 1b, JFH-1 GT 2a S282T showed only a very modest shift in 50% effective concentration (EC(50)) for PSI-7977. Sequence analysis of the JFH-1 NS5B region indicated that additional amino acid changes were selected both prior to and after the emergence of S282T. These include T179A, M289L, I293L, M434T, and H479P. Residues 179, 289, and 293 are located within the finger and palm domains, while 434 and 479 are located on the surface of the thumb domain. Data from the JFH-1 replicon variants showed that amino acid changes within the finger and palm domains together with S282T were required to confer resistance to PSI-7977, while the mutations on the thumb domain serve to enhance the replication capacity of the S282T replicons.


