PSI-7976HCV RNA replication inhibitor CAS# 1190308-01-0 |
- Tegobuvir
Catalog No.:BCC1991
CAS No.:1000787-75-6
- VX-222 (VCH-222, Lomibuvir)
Catalog No.:BCC2108
CAS No.:1026785-59-0
- R-1479
Catalog No.:BCC1878
CAS No.:478182-28-4
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Danoprevir (RG7227)
Catalog No.:BCC2106
CAS No.:850876-88-9
- RO-9187
Catalog No.:BCC1904
CAS No.:876708-03-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1190308-01-0 | SDF | Download SDF |
| PubChem ID | 45375809 | Appearance | Powder |
| Formula | C22H29FN3O9P | M.Wt | 529.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
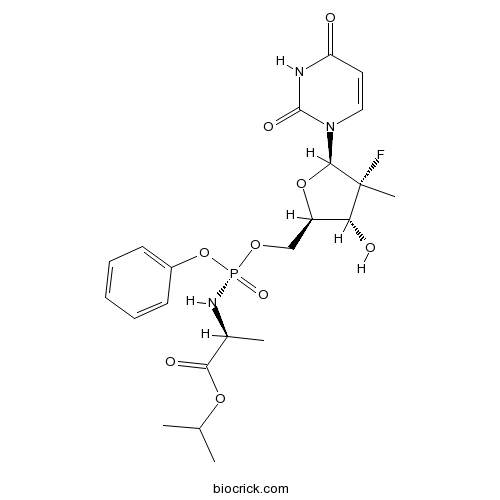
| Chemical Name | propan-2-yl (2S)-2-[[[(2R,3R,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyloxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate | ||
| SMILES | CC(C)OC(=O)C(C)NP(=O)(OCC1C(C(C(O1)N2C=CC(=O)NC2=O)(C)F)O)OC3=CC=CC=C3 | ||
| Standard InChIKey | TTZHDVOVKQGIBA-YBSJRAAASA-N | ||
| Standard InChI | InChI=1S/C22H29FN3O9P/c1-13(2)33-19(29)14(3)25-36(31,35-15-8-6-5-7-9-15)32-12-16-18(28)22(4,23)20(34-16)26-11-10-17(27)24-21(26)30/h5-11,13-14,16,18,20,28H,12H2,1-4H3,(H,25,31)(H,24,27,30)/t14-,16+,18+,20+,22+,36+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PSI-7976 is the isomer of PSI-7977. PSI-7977 is an active inhibitor of HCV RNA replication in the HCV replicon assay, demonstrates potent anti-hepatitis C virus (HCV) activity.In Vitro:PSI-7851 is a mixture of two diastereoisomers, PSI-7976 and PSI-7977, with PSI-7977 being the more active inhibitor of HCV RNA replication in the HCV replicon assay. Carboxylesterase 1 (CES1) preferentially hydrolyzes PSI-7976 over PSI-7977. The kinetic data also indicates that PSI-7976 is a better substrate for CES1 than for cathepsin A (CatA)[1] References: | |||||

PSI-7976 Dilution Calculator

PSI-7976 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8888 mL | 9.4438 mL | 18.8875 mL | 37.775 mL | 47.2188 mL |
| 5 mM | 0.3778 mL | 1.8888 mL | 3.7775 mL | 7.555 mL | 9.4438 mL |
| 10 mM | 0.1889 mL | 0.9444 mL | 1.8888 mL | 3.7775 mL | 4.7219 mL |
| 50 mM | 0.0378 mL | 0.1889 mL | 0.3778 mL | 0.7555 mL | 0.9444 mL |
| 100 mM | 0.0189 mL | 0.0944 mL | 0.1889 mL | 0.3778 mL | 0.4722 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PSI-7976 is an inhibitor of HCV RNA replication with EC50 value of 1.07μM [1].
PSI-7851 is a phosphoramidate prodrug of PSI-7411 and has potent anti-hepatitis C virus (HCV) activity. It is a mixture of two diastereoisomers. PSI-7976 is one of them. Another one is PSI-7977. PSI-7851 is firstly activated through being hydrolyzed by CatA and CES1in the liver cells. It is found that CatA prefers PSI-7977 as a substrate over PSI-7976 while CES1 preferentially hydrolyzes PSI-7976 over PSI-7977. Since CES1 does not express in clone A replicon cells, the ability of PSI-7976 and PSI-7977 to inhibit HCV RNA replication is different in these cells. PSI-7977 is more potent than PSI-7976 with EC50 value of 92nM [1].
References:
[1] Murakami E, Tolstykh T, Bao H, et al. Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977. Journal of Biological Chemistry, 2010, 285(45): 34337-34347.
- PSI-7977
Catalog No.:BCC1871
CAS No.:1190307-88-0
- Sarcandrone B
Catalog No.:BCN6074
CAS No.:1190225-48-9
- Sarcandrone A
Catalog No.:BCN6073
CAS No.:1190225-47-8
- M2 ion channel blocker
Catalog No.:BCC1726
CAS No.:1190215-03-2
- 4-[2-[(3-Ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl]benzenesulfonamide
Catalog No.:BCC8672
CAS No.:119018-29-0
- Abiesinol F
Catalog No.:BCN6418
CAS No.:1190070-91-7
- 2,2'-Biquinoline
Catalog No.:BCC8489
CAS No.:119-91-5
- 3,4-Dihydrocoumarin
Catalog No.:BCN6793
CAS No.:119-84-6
- 5-Amino-2-naphthalenesulfonic acid
Catalog No.:BCC8732
CAS No.:119-79-9
- 2-Carboxybenzaldehyde
Catalog No.:BCN2274
CAS No.:119-67-5
- Benzophenone
Catalog No.:BCC8859
CAS No.:119-61-9
- Benzoin
Catalog No.:BCC8854
CAS No.:119-53-9
- EB 47
Catalog No.:BCC2452
CAS No.:1190332-25-2
- MRT67307
Catalog No.:BCC1779
CAS No.:1190378-57-4
- Euchrenone B1
Catalog No.:BCN3575
CAS No.:119061-09-5
- Fmoc-Asp-OH
Catalog No.:BCC3085
CAS No.:119062-05-4
- Fluorobexarotene
Catalog No.:BCC6110
CAS No.:1190848-23-7
- Phellolactone
Catalog No.:BCN3467
CAS No.:1190897-23-4
- Linolenic acid ethyl ester
Catalog No.:BCN8333
CAS No.:1191-41-9
- 1-Acetoxy-5-deacetylbaccatin I
Catalog No.:BCN6357
CAS No.:119120-27-3
- IOWH-032
Catalog No.:BCC3922
CAS No.:1191252-49-9
- Ganomycin I
Catalog No.:BCN3504
CAS No.:1191255-15-8
- B-Raf inhibitor 1 dihydrochloride
Catalog No.:BCC4183
CAS No.:1191385-19-9
- 3-Oxosapriparaquinone
Catalog No.:BCN3153
CAS No.:119139-56-9
Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977.[Pubmed:20801890]
J Biol Chem. 2010 Nov 5;285(45):34337-47.
A phosphoramidate prodrug of 2'-deoxy-2'-alpha-fluoro-beta-C-methyluridine-5'-monophosphate, PSI-7851, demonstrates potent anti-hepatitis C virus (HCV) activity both in vitro and in vivo. PSI-7851 is a mixture of two diastereoisomers, PSI-7976 and PSI-7977, with PSI-7977 being the more active inhibitor of HCV RNA replication in the HCV replicon assay. To inhibit the HCV NS5B RNA-dependent RNA polymerase, PSI-7851 must be metabolized to the active triphosphate form. The first step, hydrolysis of the carboxyl ester by human cathepsin A (CatA) and/or carboxylesterase 1 (CES1), is a stereospecific reaction. Western blot analysis showed that CatA and CES1 are both expressed in primary human hepatocytes. However, expression of CES1 is undetectable in clone A replicon cells. Studies with inhibitors of CatA and/or CES1 indicated that CatA is primarily responsible for hydrolysis of the carboxyl ester in clone A cells, although in primary human hepatocytes, both CatA and CES1 contribute to the hydrolysis. Hydrolysis of the ester is followed by a putative nucleophilic attack on the phosphorus by the carboxyl group resulting in the spontaneous elimination of phenol and the production of an alaninyl phosphate metabolite, PSI-352707, which is common to both isomers. The removal of the amino acid moiety of PSI-352707 is catalyzed by histidine triad nucleotide-binding protein 1 (Hint1) to give the 5'-monophosphate form, PSI-7411. siRNA-mediated Hint1 knockdown studies further indicate that Hint1 is, at least in part, responsible for converting PSI-352707 to PSI-7411. PSI-7411 is then consecutively phosphorylated to the diphosphate, PSI-7410, and to the active triphosphate metabolite, PSI-7409, by UMP-CMP kinase and nucleoside diphosphate kinase, respectively.


