TTP 22CK2 inhibitor CAS# 329907-28-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 329907-28-0 | SDF | Download SDF |
| PubChem ID | 1536915 | Appearance | Powder |
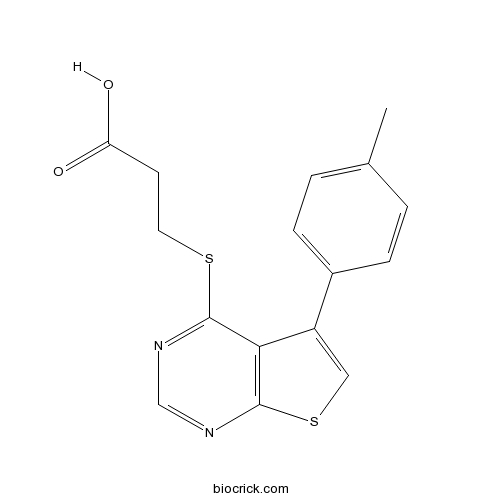
| Formula | C16H14N2O2S2 | M.Wt | 330.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 51 mg/mL (154.35 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 3-[5-(4-methylphenyl)thieno[2,3-d]pyrimidin-4-yl]sulfanylpropanoic acid | ||
| SMILES | CC1=CC=C(C=C1)C2=CSC3=C2C(=NC=N3)SCCC(=O)O | ||
| Standard InChIKey | RAOULLCLLOGTDA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14N2O2S2/c1-10-2-4-11(5-3-10)12-8-22-16-14(12)15(17-9-18-16)21-7-6-13(19)20/h2-5,8-9H,6-7H2,1H3,(H,19,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity, ATP-competitive casein kinase 2 (CK2) inhibitor (IC50 = 0.1 μM, Ki = 40 nM). Displays selectivity for CK2 over JNK3, ROCK1 and MET (no inhibitory effects at 10 μM). |

TTP 22 Dilution Calculator

TTP 22 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0265 mL | 15.1323 mL | 30.2645 mL | 60.529 mL | 75.6613 mL |
| 5 mM | 0.6053 mL | 3.0265 mL | 6.0529 mL | 12.1058 mL | 15.1323 mL |
| 10 mM | 0.3026 mL | 1.5132 mL | 3.0265 mL | 6.0529 mL | 7.5661 mL |
| 50 mM | 0.0605 mL | 0.3026 mL | 0.6053 mL | 1.2106 mL | 1.5132 mL |
| 100 mM | 0.0303 mL | 0.1513 mL | 0.3026 mL | 0.6053 mL | 0.7566 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50= 0.1 μM; Ki= 40 nM
TTP 22 is a high affinity, ATP-competitive casein kinase 2 (CK2) inhibitor.
Casein kinase 2 (CK2) noticeably stands out against a background of the kinase family due to its constitutive catalytic activity with the ability to phosphorylate more than 300 physiological substrates. These features make CK2 appear greatly diverse points of cell signaling pathways and be involved in processes leading to the development of various disorders, especially cancer. Thus, currently CK2 is regarded as druggable protein kinase target and can be used for the development of antitumor, anti-inflammatory and antiviral drugs.
In vitro: Kinetic studies of TTP 22 showed that activity of (thieno[2,3-d]pyrimidin-4-ylthio)carboxylic acids moiety was a result of its competition with ATP molecule for the binding site. Inhibition constant (Ki) for TTP 22 was 40 nM. Initial in vitro tests of TTP 22 and it analog on four serine/threonine (ASK1, JNK3, Aurora A and Rock 1) and three tyrosine protein kinases (FGFR1, Met and Tie2) revealed their remarkable specificity towards CK2 [1].
In vivo: So far, no animal in vivo study has been conducted for TTP 22.
Clinical trial: N/A
Reference:
[1] Golub AG,Bdzhola VG,Briukhovetska NV,Balanda AO,Kukharenko OP,Kotey IM,Ostrynska OV,Yarmoluk SM. Synthesis and biological evaluation of substituted (thieno[2,3-d]pyrimidin-4-ylthio)carboxylic acids as inhibitors of human protein kinase CK2. Eur J Med Chem.2011 Mar;46(3):870-6.
- Viomycin
Catalog No.:BCC3930
CAS No.:32988-50-4
- Tobramycin
Catalog No.:BCC4739
CAS No.:32986-56-4
- 10-Deacetylbaccatin III
Catalog No.:BCN5251
CAS No.:32981-86-5
- Methyl (2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoate
Catalog No.:BCN8520
CAS No.:32981-85-4
- Cinaciguat
Catalog No.:BCC1484
CAS No.:329773-35-5
- 2-Acetyl-3-ethylpyrazine
Catalog No.:BCC8512
CAS No.:32974-92-8
- Pinoresinol diacetate
Catalog No.:BCN5250
CAS No.:32971-25-8
- FPR A14
Catalog No.:BCC7498
CAS No.:329691-12-5
- 7-O-Methylaloeresin A
Catalog No.:BCN2849
CAS No.:329361-25-3
- Sanggenol L
Catalog No.:BCN3692
CAS No.:329319-20-2
- Boc-His(Trt)-OH
Catalog No.:BCC3403
CAS No.:32926-43-5
- Quetiapine hydroxy impurity
Catalog No.:BCN5340
CAS No.:329216-67-3
- 3,4-Secocucurbita-4,24-diene-3,26,29-trioic acid
Catalog No.:BCN1458
CAS No.:329975-47-5
- Nicarbazin
Catalog No.:BCC9101
CAS No.:330-95-0
- Kumatakenin
Catalog No.:BCN5252
CAS No.:3301-49-3
- ent-16beta,17-Dihydroxy-19-kauranoic acid
Catalog No.:BCN1457
CAS No.:3301-61-9
- Cyanidin-3-O-sambubioside chloride
Catalog No.:BCN3150
CAS No.:33012-73-6
- SU6656
Catalog No.:BCC6392
CAS No.:330161-87-0
- TC HSD 21
Catalog No.:BCC6228
CAS No.:330203-01-5
- Boc-β-Ala-OH
Catalog No.:BCC3051
CAS No.:3303-84-2
- Aloe-emodin-8-O-beta-D-glucopyranoside
Catalog No.:BCN1456
CAS No.:33037-46-6
- H-Orn(Z)-OH
Catalog No.:BCC3003
CAS No.:3304-51-6
- Peramivir
Catalog No.:BCC1846
CAS No.:330600-85-6
- TCTU
Catalog No.:BCC2689
CAS No.:330641-16-2
Efficacy and Safety of Vinorelbine Plus Cisplatin vs. Gemcitabine Plus Cisplatin for Treatment of Metastatic Triple-Negative Breast Cancer After Failure with Anthracyclines and Taxanes.[Pubmed:28957036]
Med Sci Monit. 2017 Sep 28;23:4657-4664.
BACKGROUND This study aimed to compare the efficacy and safety of vinorelbine plus cisplatin (NP regimen) vs. gemcitabine plus cisplatin (GP regimen) for treatment of metastatic TNBC after failure with anthracyclines and taxanes. MATERIAL AND METHODS A total of 48 patients with metastatic TNBC that failed in anthracyclines and taxanes treatment were enrolled and randomly grouped. Patients in the NP group (n=22) were given 25 mg/m(2) vinorelbine on days 1 and 8 and 25 mg/m(2) cisplatin on days 2-4 of each 21-day cycle, while subjects in the GP group (n=26) were administered 1000 mg/m(2) gemcitabine on days 1 and 8 and 25 mg/m(2) cisplatin on days 2-4 of each 21-day cycle. The treatment response and adverse events were compared between the 2 groups every 2 cycles. RESULTS The ORR, DCR, and median TTP were 45.5%, 77.3%, and 5 months in the NP group, and 46.2%, 80.8%, and 5.2 months in the GP group, and no significant differences were observed in ORR, DCR, and median TTP between the 2 groups (P>0.05). The major adverse events included grade I-II bone marrow inhibition, gastrointestinal reactions, and phlebitis, and a lower incidence of thrombocytopenia and rash and a higher incidence of phlebitis was found in the NP group than in the GP group (P<0.05). CONCLUSIONS Either NP or GP regimen is active and tolerated in treatment of metastatic TNBC with anthracyclines and/or taxanes resistance, which may be used as a salvage treatment for metastatic TNBC.
[Prenatal diagnosis of abdominal wall defects].[Pubmed:28890277]
Arch Pediatr. 2017 Oct;24(10):917-924.
Anterior abdominal wall defects (AAWD) correspond to a wide spectrum of congenital defects affecting 6.3/10,000 pregnancies. They have in common a closure defect of the anterior abdominal wall and can be fatal or expose the fetus and the neonate (NN) to many complications. This study was based on a retrospective study of 22 cases of AAWD collected between May 2009 and December 2014. Its purpose was to specify the importance of prenatal ultrasonography in the diagnosis and prognosis assessment of these defects. These 22 AAWDs consisted in 13 cases of omphalocele (including four cases of Beckwith-Wiedemann syndrome), four of gastroschisis, one of pentalogy of Cantrell, three of vesical exstrophy and one of cloacal exstrophy. Prenatal ultrasonography provided the diagnosis of 14 of these defects with a changing sensitivity with the gestational age varying from 17% in the first trimester to 71.4% and 77.8% in the second and third trimesters, respectively. The relevance of this examination was improved when performed by an imaging specialist. The prenatal diagnosis of these defects indicated an amniocentesis in eight cases, allowing the diagnosis of two cases of trisomy 18. It also motivated a therapeutic termination of the pregnancy (TTP) in ten cases. Prenatal ultrasonography allowed better prenatal follow-up and planning of the delivery of the continued pregnancies. It indicated an emergency C-section in only one case by showing intestinal complications of gastroschisis. Four NNs died (two cases of omphalocele and two of gastroschisis), three of which postoperatively and the prenatal diagnosis did not improve survival. Prenatal ultrasonographic diagnosis provided a precise morphological study determining the type of the AAWD, a complete malformation assessment, and the prognosis factors. This resulted in adequate multidisciplinary pre and postnatal care, including a rigorous ultrasound follow-up, a TTP in case of associated defects, and emergency delivery once the complications of poor diagnosis are detected.
2-Dimensional Speckle Tracking Echocardiography predicts severe coronary artery disease in women with normal left ventricular function: a case-control study.[Pubmed:28836949]
BMC Cardiovasc Disord. 2017 Aug 24;17(1):231.
BACKGROUND: Women who have coronary artery disease (CAD) often present with atypical symptoms that may lead to misdiagnosis. We assessed strain, systolic strain rate and left ventricular dyssynchrony with 2- dimensional- speckle tracking echocardiography to evaluate its use as a non-invasive method for detecting CAD in women with normal ejection fraction compared with healthy women controls with a normal angiogram. METHODS: We included 35 women with CAD confirmed by coronary angiography and a positive exercise stress echocardiography and 35 women in a control group with a low pretest probability of CAD, normal angiogram and a normal stress echocardiography with normal EF. RESULTS: Statistically significant 2D-STE findings for the CAD vs control groups were as follows for the mean of: global circumferential strain (CS) (-19.4% vs -22.4%, P = .02); global radial S (49% vs 34%, P = .03); global radial SR (2.4 s(-1) vs 1.9 s(-1), P = .05); global longitudinal LV S (GLS) (-14.3% vs -17.2%, P < .001). For mechanical dyssynchrony, SD of the GLS time-to-peak (TTP) was computed (99 vs 33 ms, P < .001). The receiver operating characteristic and area under the curve (AUC) were calculated. A cutoff value of 45 ms for 1 SD of the longitudinal S TTP had 97% sensitivity and 89% specificity (AUC, 0.96). GLS cutoff value of -15.87% had 71% sensitivity and 74% specificity; AUC, 0.74 in differentiating CAD and control groups. The combined GLS, CS, and SD of the longitudinal S TTP had an AUC of 0.96 (sensitivity 97%, specificity 86%). Interclass correlations of the GLS segment and GLS TTP measurements were 0.49 (95% CI, 0.227-0.868) and 0.74 (95% CI, 0.277-0.926), respectively. CONCLUSION: In women with a normal echocardiogram and LVEF, CAD can be identified by dyssynchrony and abnormal strain values, as evidenced by 2D-STE.
Coronary artery rotation in native and stented porcine coronary arteries.[Pubmed:28836331]
Catheter Cardiovasc Interv. 2018 May 1;91(6):1092-1100.
INTRODUCTION: Coronary arteries are exposed to several complex biomechanical forces during the cardiac cycle. These biomechanical forces potentially contribute to both native coronary artery disease, development of atherosclerosis and eventual stent failure. The aim of the present study was to characterize and define coronary artery axial rotation and the effect of stent implantation on this biomechanical factor. METHODS: Intravascular ultrasound (IVUS) images were obtained from porcine coronary arteries and analyzed in ultrasound analysis software used to evaluate myocardial strain and torsion in echocardiography. In this study the software was utilized for a novel application to evaluate coronary artery rotation and time-to-peak (TTP) rotation in porcine coronary arteries. Clockwise (CW) and counterclockwise (CCW) rotation of coronary arteries during the cardiac cycle and (TTP) rotation were measured. RESULTS: A total of 11 (4 LAD, 4 LCX, 3 RCA) coronary artery segments were independently analyzed pre- and post-stent implantation for a total of 22 IVUS runs. CW and CCW rotation and TTP varied widely within coronary artery segments and between different coronary arteries. Stent implantation impacted degree, direction and TTP of coronary rotation. Measurement reliability was assessed and the intraclass correlation coefficient for maximum average CCW was 0.990 (95% confidence interval 0.980-0.996, P < 0.0001), indicating excellent agreement. CONCLUSIONS: Coronary arteries display wide spectrum of CW and CCW rotation during the cardiac cycle. Coronary stents impact the degree and direction of coronary artery rotation. The implications of these findings on development of atherosclerosis and stent failure require further investigation.
Comparison of (18)F-FET PET and perfusion-weighted MRI for glioma grading: a hybrid PET/MR study.[Pubmed:28831534]
Eur J Nucl Med Mol Imaging. 2017 Dec;44(13):2257-2265.
PURPOSE: Both perfusion-weighted MR imaging (PWI) and O-(2-(18)F-fluoroethyl)-L-tyrosine PET ((18)F-FET) provide grading information in cerebral gliomas. The aim of this study was to compare the diagnostic value of (18)F-FET PET and PWI for tumor grading in a series of patients with newly diagnosed, untreated gliomas using an integrated PET/MR scanner. METHODS: Seventy-two patients with untreated gliomas [22 low-grade gliomas (LGG), and 50 high-grade gliomas (HGG)] were investigated with (18)F-FET PET and PWI using a hybrid PET/MR scanner. After visual inspection of PET and PWI maps (rCBV, rCBF, MTT), volumes of interest (VOIs) with a diameter of 16 mm were centered upon the maximum of abnormality in the tumor area in each modality and the contralateral unaffected hemisphere. Mean and maximum tumor-to-brain ratios (TBRmean, TBRmax) were calculated. In addition, Time-to-Peak (TTP) and slopes of time-activity curves were calculated for (18)F-FET PET. Diagnostic accuracies of (18)F-FET PET and PWI for differentiating low-grade glioma (LGG) from high-grade glioma (HGG) were evaluated by receiver operating characteristic analyses (area under the curve; AUC). RESULTS: The diagnostic accuracy of (18)F-FET PET and PWI to discriminate LGG from HGG was similar with highest AUC values for TBRmean and TBRmax of (18)F-FET PET uptake (0.80, 0.83) and for TBRmean and TBRmax of rCBV (0.80, 0.81). In case of increased signal in the tumor area with both methods (n = 32), local hot-spots were incongruent in 25 patients (78%) with a mean distance of 10.6 +/- 9.5 mm. Dynamic FET PET and combination of different parameters did not further improve diagnostic accuracy. CONCLUSIONS: Both (18)F-FET PET and PWI discriminate LGG from HGG with similar diagnostic performance. Regional abnormalities in the tumor area are usually not congruent indicating that tumor grading by (18)F-FET PET and PWI is based on different pathophysiological phenomena.
Synthesis and biological evaluation of substituted (thieno[2,3-d]pyrimidin-4-ylthio)carboxylic acids as inhibitors of human protein kinase CK2.[Pubmed:21276643]
Eur J Med Chem. 2011 Mar;46(3):870-6.
A novel series of substituted (thieno[2,3-d]pyrimidin-4-ylthio)carboxylic acids has been synthesized and tested in vitro towards human protein kinase CK2. It was revealed that the most active compounds inhibiting CK2 are 3-{[5-(4-methylphenyl)thieno[2,3-d]pyrimidin-4-yl]thio}propanoic acid and 3-{[5-(4-ethoxyphenyl)thieno[2,3-d]pyrimidin-4-yl]thio}propanoic acid (IC(50) values are 0.1 muM and 0.125 muM, respectively). Structure-activity relationships of 28 tested thienopyrimidine derivatives have been studied and binding mode of this chemical class has been predicted. Evaluation of the inhibitors on seven protein kinases revealed considerable selectivity towards CK2.


