TBBCasein kinase-2 (CK2) inhibitor CAS# 17374-26-4 |
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 17374-26-4 | SDF | Download SDF |
| PubChem ID | 1694 | Appearance | Powder |
| Formula | C6HBr4N3 | M.Wt | 434.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC 231634 | ||
| Solubility | DMSO : ≥ 100 mg/mL (230.04 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
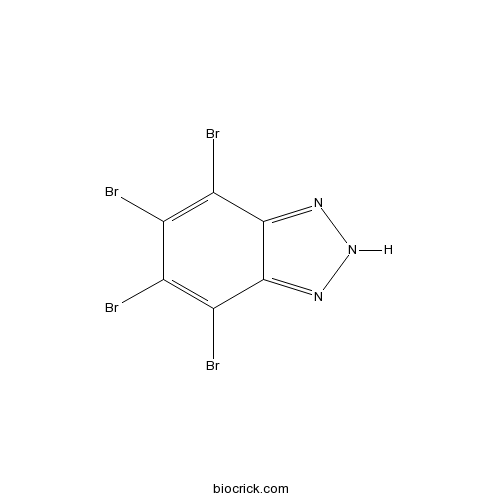
| Chemical Name | 4,5,6,7-tetrabromo-2H-benzotriazole | ||
| SMILES | C1(=C(C2=NNN=C2C(=C1Br)Br)Br)Br | ||
| Standard InChIKey | OMZYUVOATZSGJY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6HBr4N3/c7-1-2(8)4(10)6-5(3(1)9)11-13-12-6/h(H,11,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A cell-permeable, selective inhibitor of casein kinase-2 (CK2) (IC50 = 0.9 and 1.6 μM for rat liver and human recombinant CK2 respectively). Exhibits modest discrimination between CK2 subunits, with Ki values ranging from 80 nM to 210 nM. Acts in an ATP/GTP-competitive manner and displays one to two orders of magnitude selectivity over a panel of 33 protein kinases. |

TBB Dilution Calculator

TBB Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3004 mL | 11.5019 mL | 23.0038 mL | 46.0077 mL | 57.5096 mL |
| 5 mM | 0.4601 mL | 2.3004 mL | 4.6008 mL | 9.2015 mL | 11.5019 mL |
| 10 mM | 0.23 mL | 1.1502 mL | 2.3004 mL | 4.6008 mL | 5.751 mL |
| 50 mM | 0.046 mL | 0.23 mL | 0.4601 mL | 0.9202 mL | 1.1502 mL |
| 100 mM | 0.023 mL | 0.115 mL | 0.23 mL | 0.4601 mL | 0.5751 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
TBB is a highly selective, ATP/GTP-competitive inhibitor of casein kinase-2 (CK2) (IC50 = 0.9 and 1.6 μM for rat liver and human recombinant CK2 respectively). TBB exhibits modest discrimination between CK2 subunits, with Ki values ranging from 80 nM to 210 nM. TBB acts in an ATP/GTP-competitive manner and displays one to two orders of magnitude selectivity over a panel of 33 protein kinases. The effect of TBB on PC-3 human prostate cancer cell viability depends on the time schedule of administration.
- 3-Galloylquinic acid
Catalog No.:BCN3732
CAS No.:17365-11-6
- Desoxygambogenin
Catalog No.:BCN3068
CAS No.:173614-93-2
- 2,3-Didehydrosomnifericin
Catalog No.:BCN8005
CAS No.:173614-88-5
- Oxotremorine sesquifumarate
Catalog No.:BCC6814
CAS No.:17360-35-9
- 3,5-Dinitro-Tyr-OH
Catalog No.:BCC3331
CAS No.:17360-11-1
- Compound W
Catalog No.:BCC2341
CAS No.:173550-33-9
- Naphthoquine phosphate
Catalog No.:BCC1784
CAS No.:173531-58-3
- HMN-214
Catalog No.:BCC2517
CAS No.:173529-46-9
- Ficusin A
Catalog No.:BCN1115
CAS No.:173429-83-9
- Rotundanonic acid
Catalog No.:BCN7152
CAS No.:173357-19-2
- Afobazole
Catalog No.:BCC5386
CAS No.:173352-21-1
- Pelargonidin-3,5-O-diglucoside chloride
Catalog No.:BCN1527
CAS No.:17334-58-6
- BGC 20-761
Catalog No.:BCC7650
CAS No.:17375-63-2
- Gambogin
Catalog No.:BCN3069
CAS No.:173792-67-1
- H-Gly-OBzl.HCl
Catalog No.:BCC2949
CAS No.:1738-68-7
- H-Ser-OBzl.HCl
Catalog No.:BCC3030
CAS No.:1738-72-3
- H-Gly-OBzl.TosOH
Catalog No.:BCC2948
CAS No.:1738-76-7
- H-Leu-OBzl.TosOH
Catalog No.:BCC2970
CAS No.:1738-77-8
- Swertiamarin
Catalog No.:BCN1116
CAS No.:17388-39-5
- Y-39983 dihydrochloride
Catalog No.:BCC4186
CAS No.:173897-44-4
- FR 171113
Catalog No.:BCC7734
CAS No.:173904-50-2
- Isocarapanaubine
Catalog No.:BCN1117
CAS No.:17391-09-2
- Corynoxine B
Catalog No.:BCN8454
CAS No.:17391-18-3
- Gambogenic acid
Catalog No.:BCN3077
CAS No.:173932-75-7
Estimation of human percutaneous bioavailability for two novel brominated flame retardants, 2-ethylhexyl 2,3,4,5-tetrabromobenzoate (EH-TBB) and bis(2-ethylhexyl) tetrabromophthalate (BEH-TEBP).[Pubmed:27732871]
Toxicol Appl Pharmacol. 2016 Nov 15;311:117-127.
2-Ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) and bis(2-ethylhexyl)tetrabromophthalate (BEH-TEBP) are novel brominated flame retardants used in consumer products. A parallelogram approach was used to predict human dermal absorption and flux for EH-TBB and BEH-TEBP. [(14)C]-EH-TBB or [(14)C]-BEH-TEBP was applied to human or rat skin at 100nmol/cm(2) using a flow-through system. Intact rats received analogous dermal doses. Treated skin was washed and tape-stripped to remove "unabsorbed" [(14)C]-radioactivity after continuous exposure (24h). "Absorbed" was quantified using dermally retained [(14)C]-radioactivity; "penetrated" was calculated based on [(14)C]-radioactivity in media (in vitro) or excreta+tissues (in vivo). Human skin absorbed EH-TBB (24+/-1%) while 0.2+/-0.1% penetrated skin. Rat skin absorbed more (51+/-10%) and was more permeable (2+/-0.5%) to EH-TBB in vitro; maximal EH-TBB flux was 11+/-7 and 102+/-24pmol-eq/cm(2)/h for human and rat skin, respectively. In vivo, 27+/-5% was absorbed and 13% reached systemic circulation after 24h (maximum flux was 464+/-65pmol-eq/cm(2)/h). BEH-TEBP in vitro penetrance was minimal (<0.01%) for rat or human skin. BEH-TEBP absorption was 12+/-11% for human skin and 41+/-3% for rat skin. In vivo, total absorption was 27+/-9%; 1.2% reached systemic circulation. In vitro maximal BEH-TEBP flux was 0.3+/-0.2 and 1+/-0.3pmol-eq/cm(2)/h for human and rat skin; in vivo maximum flux for rat skin was 16+/-7pmol-eq/cm(2)/h. EH-TBB was metabolized in rat and human skin to tetrabromobenzoic acid. BEH-TEBP-derived [(14)C]-radioactivity in the perfusion media could not be characterized. <1% of the dose of EH-TBB and BEH-TEHP is estimated to reach the systemic circulation following human dermal exposure under the conditions tested. CHEMICAL COMPOUNDS STUDIED IN THIS ARTICLE: 2-Ethylhexyl 2,3,4,5-tetrabromobenzoate (PubChem CID: 71316600; CAS No. 183658-27-7 FW: 549.92g/mol logPest: 7.73-8.75 (12)) Abdallah et al., 2015a. Other published abbreviations for 2-ethylhexyl-2,3,4,5-tetrabromobenzoate are TBB EHTeBB or EHTBB Abdallah and Harrad, 2011. bis(2-ethylhexyl) tetrabromophthalate (PubChem CID: 117291; CAS No. 26040-51-7 FW: 706.14g/mol logPest: 9.48-11.95 (12)). Other published abbreviations for bis(2-ethylhexyl)tetrabromophthalate are TeBrDEPH TBPH or BEHTBP.
Diagnosis of resistance alleles in codon 167 of the beta-tubulin (Cya-tbb-1) gene from third-stage larvae of horse cyathostomins.[Pubmed:28199900]
Res Vet Sci. 2017 Dec;115:92-95.
Anthelmintic resistance is a serious problem for the control of equine gastrointestinal nematodes. In the present survey, 173 third stage larvae of cyathostomins were investigated from three different locations for the presence of the resistant genotype at codon 167 of the beta-tubulin gene, as this is the most prevalent mutation. The larvae from the state of Parana (n=67), Sao Paulo (n=54) and Santa Catarina (n=52), showed 61.2; 31.5 and 38.5% of the heterozygous resistant genotype - TTC/TAC, respectively. An unpublished mutation at codon 172 that results in a serine (S) to threonine (T) substitution was found in 17.9% (12/67) of samples from Parana; and 13.0% (7/54) of samples from Sao Paulo. We have compared the molecular diagnostic with the fecal egg count data (R(2)=-0.79) from the same farms, and consider that the use of routine molecular diagnostic in individual larva may help to determine the population genetic distribution that is associated with drug failure.
Effects of 4-META/MMA-TBB Resin at Different Curing Stages on Osteoblasts and Gingival Epithelial Cells.[Pubmed:27022642]
J Adhes Dent. 2016;18(2):111-8.
PURPOSE: To assess the effects of different curing stages of 4-META/MMA-TBB resin on osteoblasts and gingival keratinocytes. MATERIALS AND METHODS: The MC3T3-E1 murine pre-osteoblastic cell line and GE-1 murine gingival epithelial cell line were cultured with mixtures of Super-Bond C&B at different curing stages, and the cell viability was assessed. The alkaline phosphatase (ALP) activity of the MC3T3-E1 cells was also assessed. RESULTS: The majority of the MC3T3-E1 cells died and showed no ALP activity when cultured with 4-META/MMA-TBB resin during the initial curing phase (1 min of curing). A later curing phase of the 4-META/MMA-TBB resin (7 min of curing) showed cytotoxicity at day 1, but the toxic effect was temporary and the proliferative capacity and ALP activity in the cells were similar to control cells at day 7. Completely cured 4-META/MMA-TBB resin (after 1 or 12 h of curing) did not affect the cell viability or ALP activity of the MC3T3-E1 cells. In contrast, 4-META/MMA-TBB resin showed no effect on the GE-1 cells at any stage of curing. CONCLUSION: Although 4-META/MMA-TBB resin during the initial curing phase shows toxic effects on MC3T3-E1 cells, that cytotoxicity is minimal at later curing phases. In contrast, neither the uncured nor cured resins affected the GE-1 cells.
Comparison of in vitro hormone activities of novel flame retardants TBB, TBPH and their metabolites TBBA and TBMEPH using reporter gene assays.[Pubmed:27380226]
Chemosphere. 2016 Oct;160:244-51.
The anti-androgenic and anti-thyroid hormonal activities of the two novel brominated flame retardants, TBB and TBPH and of their metabolites TBBA and TBMEPH have been compared using the luciferase reporter gene assays. Only the parent compounds TBB and TBPH exhibited anti-glucocorticoid activity with IC50 values of 1.9 muM and 0.3 muM. Furthermore, mode of action for these two compounds is by direct competing to the glucocorticoid receptor (GR) with IC50 values of 0.03 muM and 0.002 muM. All four tested compounds possess anti-androgenic and anti-thyroid hormonal activities, without agonist activities on the respective receptors. Anti-androgenic activities with IC50 values of 43.5 muM, 0.1 muM, 47.5 muM and 1.3 muM were found for TBB, TBPH, TBBA and TBMEPH. The anti-thyroid hormonal IC50 values of 37.5 muM, 0.1 muM, 22.8 muM and 32.3 muM for TBB, TBPH, TBBA and TBMEPH, together with the above quoted results, indicate that metabolism can modify anti-androgenic, anti-glucocorticoid and anti-thyroid hormonal effects of these novel brominated flame retardants. Furthermore, the parent flame retardants are shown to be able to disrupt the function of the GR as antagonists by direct competition to the receptor.
Tetrabromobenzotriazole (TBBt) and tetrabromobenzimidazole (TBBz) as selective inhibitors of protein kinase CK2: evaluation of their effects on cells and different molecular forms of human CK2.[Pubmed:16203192]
Biochim Biophys Acta. 2005 Dec 30;1754(1-2):271-80.
The development of selective cell-permeable inhibitors of protein kinase CK2 has represented an important advance in the field. However, it is important to not overlook the existence of discrete molecular forms of CK2 that arise from the presence of distinct isozymic forms, and the existence of the catalytic CK2 subunits as free subunits and in complexes with the regulatory CK2beta subunits and, possibly, other proteins. This review examines two recently developed, and presently widely applied, CK2 inhibitors, 4,5,6,7-tetrabromobenzotriazole (TBBt) and the related 4,5,6,7-tetrabromobenzimidazole (TBBz), the latter of which was previously shown to discriminate between different molecular forms of CK2 in yeast. We have shown, by spectrophotometric titration, that TBBt, with a pK(a) approximately 5, exists in solution at physiological pH almost exclusively (>99%) as the monoanion; whereas TBBz, with a pKa approximately 9, is predominantly (>95%) in the neutral form, both of obvious relevance to their modes of binding. In vitro, TBBt inhibits different forms of CK2 with Ki values ranging from 80 to 210 nM. TBBz better discriminates between CK2 forms, with Ki values ranging from 70 to 510 nM. Despite their general similar in vitro activities, TBBz is more effective than TBBt in inducing apoptosis and, to a lesser degree, necrosis, in transformed human cell lines. Finally, development of shRNA strategies for the selective knockdown of the CK2alpha and CK2alpha' isoforms reinforces the foregoing results, indicating that inhibition of CK2 leads to attenuation of proliferation.
Optimization of protein kinase CK2 inhibitors derived from 4,5,6,7-tetrabromobenzimidazole.[Pubmed:15566294]
J Med Chem. 2004 Dec 2;47(25):6239-47.
Casein kinase 2 (CK2) is a ubiquitous, essential, and highly pleiotropic protein kinase whose abnormally high constitutive activity is suspected to underlie its pathogenic potential in neoplasia and infective diseases. Thus, CK2 inhibitors designed to dissect the signaling pathways affected by this kinase, in perspective, may give rise to pharmacological tools. One of the most successful CK2 inhibitors is TBB (4,5,6,7-tetrabromobenzotriazole). Here we show that its inhibitory properties can be markedly improved by generating adducts in which N(2) is replaced by a carbon atom bound to a variety of polar functions. The most efficient inhibitor is 4,5,6,7-tetrabromo-2-(dimethylamino)benzimidazole (2c) followed by the methylsulfanyl (8), isopropylamino (2e), and amino (2a) congeners. All these compounds display K(i) values <100 nM (40 nM in the case of 2c). 2c induces apoptosis of Jurkat cells more readily than TBB (DC(50) value 2.7 vs 17 microM) and, unlike TBB, it does not display any side effect on mitochondria polarization up to 10 microM concentration. Molecular modeling of the CK2-2c complex, based on the crystal structure of the CK2-TBB complex suggests that a number of additional apolar contacts between its two methyl groups and hydrophobic residues nearby could account for its superior inhibitory properties. Consequently, 2c is even more susceptible than TBB to mutations of the unique hydrophobic residues V66 and/or I174 to alanine. We propose to adopt 2c as first choice CK2 inhibitor instead of TBB, especially for in cell studies.
Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 ('casein kinase-2').[Pubmed:11343704]
FEBS Lett. 2001 May 4;496(1):44-8.
The specificity of 4,5,6,7-tetrabromo-2-azabenzimidazole (TBB), an ATP/GTP competitive inhibitor of protein kinase casein kinase-2 (CK2), has been examined against a panel of 33 protein kinases, either Ser/Thr- or Tyr-specific. In the presence of 10 microM TBB (and 100 microM ATP) only CK2 was drastically inhibited (>85%) whereas three kinases (phosphorylase kinase, glycogen synthase kinase 3 beta and cyclin-dependent kinase 2/cyclin A) underwent moderate inhibition, with IC(50) values one--two orders of magnitude higher than CK2 (IC(50)=0.9 microM). TBB also inhibits endogenous CK2 in cultured Jurkat cells. A CK2 mutant in which Val66 has been replaced by alanine is much less susceptible to inhibition by TBB as well as by another ATP competitive inhibitor, emodin. These data show that TBB is a quite selective inhibitor of CK2, that can be used in cell-based assays.


