HMN-214Plk inhibitor,broad-spectrum anti-tumor agent CAS# 173529-46-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 173529-46-9 | SDF | Download SDF |
| PubChem ID | 9888590 | Appearance | Powder |
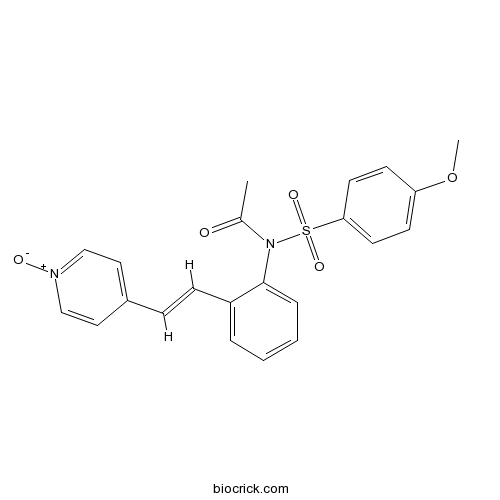
| Formula | C22H20N2O5S | M.Wt | 424.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | IVX-214 | ||
| Solubility | DMSO : 25 mg/mL (58.90 mM; Need ultrasonic) | ||
| Chemical Name | N-(4-methoxyphenyl)sulfonyl-N-[2-[(E)-2-(1-oxidopyridin-1-ium-4-yl)ethenyl]phenyl]acetamide | ||
| SMILES | CC(=O)N(C1=CC=CC=C1C=CC2=CC=[N+](C=C2)[O-])S(=O)(=O)C3=CC=C(C=C3)OC | ||
| Standard InChIKey | OCKHRKSTDPOHEN-BQYQJAHWSA-N | ||
| Standard InChI | InChI=1S/C22H20N2O5S/c1-17(25)24(30(27,28)21-11-9-20(29-2)10-12-21)22-6-4-3-5-19(22)8-7-18-13-15-23(26)16-14-18/h3-16H,1-2H3/b8-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | HMN-214, a prodrug of HMN-176, ispotent and broad-spectrumanti-tumor reagent. | |||||
| Targets | PLK1 | |||||

HMN-214 Dilution Calculator

HMN-214 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3559 mL | 11.7794 mL | 23.5588 mL | 47.1176 mL | 58.897 mL |
| 5 mM | 0.4712 mL | 2.3559 mL | 4.7118 mL | 9.4235 mL | 11.7794 mL |
| 10 mM | 0.2356 mL | 1.1779 mL | 2.3559 mL | 4.7118 mL | 5.8897 mL |
| 50 mM | 0.0471 mL | 0.2356 mL | 0.4712 mL | 0.9424 mL | 1.1779 mL |
| 100 mM | 0.0236 mL | 0.1178 mL | 0.2356 mL | 0.4712 mL | 0.589 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: HMN-176 showed potent cytotoxic activity against several tumor cell lines with an average IC50 of 118 nmol/L
The polo-like kinases (PLK) are a group of highly conserved serine/threonine kinases that serve as regulatory enzymes for mitotic events. HMN-214 is an oral prodrug of HMN-176, a stilbene derivative that interferes with the subcellular spatial location of polo-like kinase-1, a serine/threonine kinase that regulates critical mitotic events.
In vitro: HMN-176 showed potent cytotoxicity, with a mean IC50 of 118 nM. The cytotoxic effecacy of HNM-176 was superior to that of ADM, VP-16 and CDDP, but inferior to that of taxol and VCR. HMN-176 showed less vaiance in log(IC50) than did the reference agents. In addition, judging by its low resistance indices, HMN-176 was more cytotoxic toward the drug-resistant phenotypes of tumor cells than were the other agents tested [1].
In vivo: PK studies showed that HNM-214 was an acceptable oral prodrug of HMN-176. In the in vivo analysis of the schedule-dependency of HMN-214, the repeated administration for over 5 days elicited potent antitumor activity, as expected from the exposure-dependency of the cytotoxicity of HMN-176 and from the cytometric studies. The antitumor activity of HMN-214 against human tumor xenografts was equal or superior to that of clinically avaiable agents, including cis-platinum, adriamycin, vincristine and UFT without severe toxicity such as neurotoxicity [1].
Clinical trial: A phase I pharmacokinetic study indicated that there was no accumulation of HMN-176, the metabolite of HMN-214, with repeated dosing. Seven of 29 patients had stable disease, including 6-month stable disease in a heavily pretreated breast cancer patient. A transient carcinoembryonic antigen decline in a patient with colorectal cancer was noted [2].
Reference:
[1] Takagi M, Honmura T, Watanabe S, Yamaguchi R, Nogawa M, Nishimura I, Katoh F, Matsuda M, Hidaka H. In vivo antitumor activity of a novel sulfonamide, HMN-214, against human tumor xenografts in mice and the spectrum of cytotoxicity of its active metabolite, HMN-176. Invest New Drugs. 2003 Nov;21(4):387-99.
[2] Garland LL, Taylor C, Pilkington DL, Cohen JL, Von Hoff DD. A phase I pharmacokinetic study of HMN-214, a novel oral stilbene derivative with polo-like kinase-1-interacting properties, in patients with advanced solid tumors. Clin Cancer Res. 2006 Sep 1;12(17):5182-9.
- Ficusin A
Catalog No.:BCN1115
CAS No.:173429-83-9
- Rotundanonic acid
Catalog No.:BCN7152
CAS No.:173357-19-2
- Afobazole
Catalog No.:BCC5386
CAS No.:173352-21-1
- Pelargonidin-3,5-O-diglucoside chloride
Catalog No.:BCN1527
CAS No.:17334-58-6
- Aliskiren Hemifumarate
Catalog No.:BCC5018
CAS No.:173334-58-2
- Aliskiren
Catalog No.:BCC1338
CAS No.:173334-57-1
- Isorhamnetin 3-glucoside-7-rhamnoside
Catalog No.:BCN1528
CAS No.:17331-71-4
- Garciniaxanthone E
Catalog No.:BCN1114
CAS No.:173294-74-1
- TC-E 5003
Catalog No.:BCC8008
CAS No.:17328-16-4
- 2,5-Dihydroxy-1-methoxyxanthone
Catalog No.:BCN7577
CAS No.:173220-32-1
- Broussonetine A
Catalog No.:BCN2515
CAS No.:173220-07-0
- Clomipramine HCl
Catalog No.:BCC5036
CAS No.:17321-77-6
- Naphthoquine phosphate
Catalog No.:BCC1784
CAS No.:173531-58-3
- Compound W
Catalog No.:BCC2341
CAS No.:173550-33-9
- 3,5-Dinitro-Tyr-OH
Catalog No.:BCC3331
CAS No.:17360-11-1
- Oxotremorine sesquifumarate
Catalog No.:BCC6814
CAS No.:17360-35-9
- 2,3-Didehydrosomnifericin
Catalog No.:BCN8005
CAS No.:173614-88-5
- Desoxygambogenin
Catalog No.:BCN3068
CAS No.:173614-93-2
- 3-Galloylquinic acid
Catalog No.:BCN3732
CAS No.:17365-11-6
- TBB
Catalog No.:BCC1988
CAS No.:17374-26-4
- BGC 20-761
Catalog No.:BCC7650
CAS No.:17375-63-2
- Gambogin
Catalog No.:BCN3069
CAS No.:173792-67-1
- H-Gly-OBzl.HCl
Catalog No.:BCC2949
CAS No.:1738-68-7
- H-Ser-OBzl.HCl
Catalog No.:BCC3030
CAS No.:1738-72-3
A phase I pharmacokinetic study of HMN-214, a novel oral stilbene derivative with polo-like kinase-1-interacting properties, in patients with advanced solid tumors.[Pubmed:16951237]
Clin Cancer Res. 2006 Sep 1;12(17):5182-9.
PURPOSE: HMN-214 is an oral prodrug of HMN-176, a stilbene derivative that interferes with the subcellular spatial location of polo-like kinase-1, a serine/threonine kinase that regulates critical mitotic events. We conducted a dose escalation study of HMN-214 in patients with advanced cancer to assess the safety profile and pharmacokinetics of HMN-214 and to establish the maximum tolerated dose. EXPERIMENTAL DESIGN: Thirty-three patients were enrolled onto four dosing cohorts of HMN-214 from 3 to 9.9 mg/m2/d using a continuous 21-day dosing schedule every 28 days, with pharmacokinetic sampling during cycle 1. RESULTS: A severe myalgia/bone pain syndrome and hyperglycemia were dose-limiting toxicities at 9.9 mg/m2/d. A dose reduction and separate enrollment by pretreatment status (lightly versus heavily pretreated) was undertaken, with one dose-limiting toxicity (grade 3 bone pain) at 8 mg/m2/d. The maximum tolerated dose was defined as 8 mg/m2/d for both treatment cohorts. Dose-proportional increases were observed in AUC but not Cmax. There was no accumulation of HMN-176, the metabolite of HMN-214, with repeated dosing. Seven of 29 patients had stable disease as best tumor response, including 6-month stable disease in a heavily pretreated breast cancer patient. A transient decline in carcinoembryonic antigen in a patient with colorectal cancer was noted. CONCLUSIONS: The maximum tolerated dose and recommended phase II dose of HMN-214 when administered on this schedule was 8 mg/m2/d regardless of pretreatment status. Further development of HMN-214 will focus on patient populations for which high expression of polo-like kinase-1 is seen (i.e., prostate and pancreatic cancer patients).
In vivo antitumor activity of a novel sulfonamide, HMN-214, against human tumor xenografts in mice and the spectrum of cytotoxicity of its active metabolite, HMN-176.[Pubmed:14586206]
Invest New Drugs. 2003 Nov;21(4):387-99.
The cytotoxic effects of HMN-176 ((E)-4-[[2-N-[4-methoxybenzenesulfonyl] amino] stilbazole] 1-oxide; a newly synthesized compound, were evaluated and compared with those of the clinically used antitumor agents cis-platinum, adriamycin, etoposide, taxol, and vincristine in 22 human tumor cell lines isolated from various organs. HMN-176 exhibited potent cytotoxicity with IC(50) values in the nM range, and the variance of its cytotoxic efficacy was remarkably small. Drug-resistant cell lines also showed low cross-resistance to HMN-176 corresponding to overall resistance indices of less than 14.3. HMN-214 was synthesized as an oral prodrug because of the poor oral absorption of HMN-176 itself. Pharmacokinetic studies showed that HMN-214 was an acceptable oral prodrug of HMN-176. In the in vivo analysis of the schedule-dependency of HMN-214, the repeated administration for over 5 days elicited potent antitumor activity, as expected from the exposure-dependency of the cytotoxicity of HMN-176 and from the cytometric studies. The antitumor activity of HMN-214 against human tumor xenografts was equal or superior to that of clinically available agents, including cis-platinum, adriamycin, vincristine, and UFT without severe toxicity such as neurotoxicity. Because of its good activity in preclinical trials, HMN-214 has entered Phase I clinical trials in the USA.
HMN-176, an active metabolite of the synthetic antitumor agent HMN-214, restores chemosensitivity to multidrug-resistant cells by targeting the transcription factor NF-Y.[Pubmed:14583495]
Cancer Res. 2003 Oct 15;63(20):6942-7.
HMN-176 ((E)-4-[[2-N-[4-methoxybenzenesulfonyl]amino]stilbazole]1-oxide) is an active metabolite of HMN-214 ((E)-4-[2-[2-(N-acetyl-N-[4-methoxybenzenesulfonyl]amino)stilbazole]]1-oxide), which has a potent antitumor activity in mouse xenograft models. In this study, we show that HMN-176 circumvents multidrug resistance in a K2 human ovarian cancer subline selected for Adriamycin resistance (K2/ARS). Upon treatment of K2/ARS cells with 3 microM HMN-176, the GI(50) of Adriamycin for the cells decreased by approximately 50%. To explore the molecular mechanism of this effect, we assessed the expression of the multidrug resistance gene (MDR1), which is constitutive in K2/ARS cells, at both the protein and the mRNA level. Western and reverse transcription-PCR analysis revealed that the expression of MDR1 was significantly suppressed by treatment with HMN-176. Furthermore, when administered p.o., HMN-214 suppressed the expression of MDR1 mRNA in a mouse xenograft model implanted with KB-A.1, an Adriamycin-resistant cell line. Luciferase reporter fusion gene analysis demonstrated that HMN-176 inhibited the Y-box-dependent promoter activity of the MDR1 gene in a dose-dependent manner. Moreover, we show by electrophoretic mobility shift assay that HMN-176 inhibits the binding of NF-Y, which is thought to be an essential factor for the basal expression of MDR1, to its target Y-box consensus sequence in the MDR-1 promoter. Inhibition of MDR-1 expression was achieved with pharmacological concentrations of HMN-176, suggesting that HMN-176 may act by two different mechanisms-cytotoxicity and MDR1 down-regulation-simultaneously. The data presented strongly suggest that the antitumor mechanism of HMN-176 (or its prodrug HMN-214 in vivo) is quite different from those of known antitumor agents.


