AliskirenDirect renin inhibitor CAS# 173334-57-1 |
- Guanfacine hydrochloride
Catalog No.:BCC1609
CAS No.:29110-48-3
- (R,R)-Formoterol
Catalog No.:BCC1293
CAS No.:67346-49-0
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
- Medetomidine
Catalog No.:BCC1736
CAS No.:86347-14-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 173334-57-1 | SDF | Download SDF |
| PubChem ID | 5493444 | Appearance | Powder |
| Formula | C30H53N3O6 | M.Wt | 551.77 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CGP 60536; CGP60536B; SPP 100 | ||
| Solubility | DMSO : 100 mg/mL (181.24 mM; Need ultrasonic) | ||
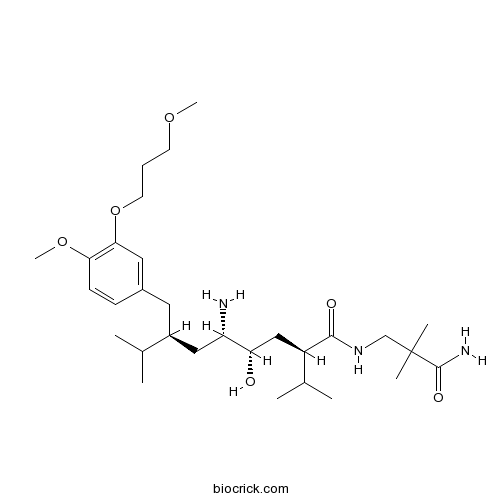
| Chemical Name | (2S,4S,5S,7S)-5-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-7-[[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl]-8-methyl-2-propan-2-ylnonanamide | ||
| SMILES | CC(C)C(CC1=CC(=C(C=C1)OC)OCCCOC)CC(C(CC(C(C)C)C(=O)NCC(C)(C)C(=O)N)O)N | ||
| Standard InChIKey | UXOWGYHJODZGMF-QORCZRPOSA-N | ||
| Standard InChI | InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Aliskiren(CGP 60536) is a direct renin inhibitor with IC50 of 1.5 nM.
IC50 value: 1.5 nM [1]
Target: renin
in vitro: Aliskiren hemifumarate appears to bind to both the hydrophobic S1/S3-binding pocket and to a large, distinct subpocket that extends from the S3-binding site towards the hydrophobic core of renin. Oral bioavailability of Aliskiren hemifumarate is 2.4% in rats, 16% in marmosets and about 2.5% in humans [2].
in vivo: Aliskiren hemifumarate (< 10 mg/kg, oral) inhibits plasma renin activity and lowers blood pressure in sodium-depleted marmosets[3].Once-daily oral treatment with Aliskiren hemifumarate lowers blood pressure effectively, with a safety and tolerability profile, in patients with mild-to-moderate hypertension[4]. References: | |||||

Aliskiren Dilution Calculator

Aliskiren Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8123 mL | 9.0617 mL | 18.1235 mL | 36.247 mL | 45.3087 mL |
| 5 mM | 0.3625 mL | 1.8123 mL | 3.6247 mL | 7.2494 mL | 9.0617 mL |
| 10 mM | 0.1812 mL | 0.9062 mL | 1.8123 mL | 3.6247 mL | 4.5309 mL |
| 50 mM | 0.0362 mL | 0.1812 mL | 0.3625 mL | 0.7249 mL | 0.9062 mL |
| 100 mM | 0.0181 mL | 0.0906 mL | 0.1812 mL | 0.3625 mL | 0.4531 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Aliskiren (trade names Tekturna, U.S.; Rasilez, U.K. and elsewhere) is the first in a class of drugs called direct renin inhibitors.Aliskiren current licensed indication is essential (primary) hypertension.
- Isorhamnetin 3-glucoside-7-rhamnoside
Catalog No.:BCN1528
CAS No.:17331-71-4
- Garciniaxanthone E
Catalog No.:BCN1114
CAS No.:173294-74-1
- TC-E 5003
Catalog No.:BCC8008
CAS No.:17328-16-4
- 2,5-Dihydroxy-1-methoxyxanthone
Catalog No.:BCN7577
CAS No.:173220-32-1
- Broussonetine A
Catalog No.:BCN2515
CAS No.:173220-07-0
- Clomipramine HCl
Catalog No.:BCC5036
CAS No.:17321-77-6
- 4-Beta-Hydroxycholesterol
Catalog No.:BCN2752
CAS No.:17320-10-4
- SQ 22536
Catalog No.:BCC7065
CAS No.:17318-31-9
- Centaureidin
Catalog No.:BCN2575
CAS No.:17313-52-9
- Ganoderic acid DM
Catalog No.:BCN1113
CAS No.:173075-45-1
- Rhoifolin
Catalog No.:BCN1112
CAS No.:17306-46-6
- Rec 15/2615 dihydrochloride
Catalog No.:BCC7628
CAS No.:173059-17-1
- Aliskiren Hemifumarate
Catalog No.:BCC5018
CAS No.:173334-58-2
- Pelargonidin-3,5-O-diglucoside chloride
Catalog No.:BCN1527
CAS No.:17334-58-6
- Afobazole
Catalog No.:BCC5386
CAS No.:173352-21-1
- Rotundanonic acid
Catalog No.:BCN7152
CAS No.:173357-19-2
- Ficusin A
Catalog No.:BCN1115
CAS No.:173429-83-9
- HMN-214
Catalog No.:BCC2517
CAS No.:173529-46-9
- Naphthoquine phosphate
Catalog No.:BCC1784
CAS No.:173531-58-3
- Compound W
Catalog No.:BCC2341
CAS No.:173550-33-9
- 3,5-Dinitro-Tyr-OH
Catalog No.:BCC3331
CAS No.:17360-11-1
- Oxotremorine sesquifumarate
Catalog No.:BCC6814
CAS No.:17360-35-9
- 2,3-Didehydrosomnifericin
Catalog No.:BCN8005
CAS No.:173614-88-5
- Desoxygambogenin
Catalog No.:BCN3068
CAS No.:173614-93-2
The renin inhibitor aliskiren protects rat lungs from the histopathologic effects of fat embolism.[Pubmed:28107310]
J Trauma Acute Care Surg. 2017 Feb;82(2):338-344.
BACKGROUND: Fat embolism (FE) and the consequent FE syndrome occurring after trauma or surgery can lead to serious pulmonary injury, including ARDS and death. Current treatment of FE syndrome is limited to supportive therapy. We have shown in a rat model that the renin angiotensin system plays a significant role in the pathophysiology of FE because drugs interfering with the renin angiotensin system, captopril and losartan reduce the histopathologic pulmonary damage. The purpose of the current study was to determine if inhibition of renin by Aliskiren, an FDA-approved drug for treating hypertension, would produce effective protection in the same model. METHODS: The FE model used intravenous injection of the neutral fat triolein in unanesthetized rats. Intraperitoneal injections of saline or Aliskiren at either 50 or 100 mg/kg were performed 1 hour after FE induction via triolein. Rats were euthanized at 48 hours, and various histologic stains were used to examine the lungs. RESULTS: (1) Fibrosis: rats treated with triolein showed significant fibrotic changes with increased collagen and myofibroblast activation (p < 0.0001 for both trichrome and alpha-smooth muscle actin staining). Aliskiren blocked this inflammatory and profibrotic process to a level indistinguishable from the controls (p < 0.0001 for both trichrome and alpha-smooth muscle actin staining). (2) Fat: rats treated with triolein showed a statistically significant increase in fat (p = 0.0006). Subsequent Aliskiren administration at both doses reduced the size, distribution, and amount of fat droplets (low dose, p = 0.0095; high dose, p = 0.0028). (3) Vessel patency: the low dose of Aliskiren blocked the reduction of lumen patency observed after triolein administration (p = 0.0058). CONCLUSIONS: Aliskiren protected the lungs of rats from gross and histopathologic FE-induced pulmonary damage at 48 hours. Clinical implications include the use of Aliskiren both prophylactically (before certain orthopedic procedures) and therapeutically (after severe trauma) to prevent the consequent severe pulmonary pathologic sequelae.
Aliskiren increases aquaporin-2 expression and attenuates lithium-induced nephrogenic diabetes insipidus.[Pubmed:28228402]
Am J Physiol Renal Physiol. 2017 Oct 1;313(4):F914-F925.
The direct renin inhibitor Aliskiren has been shown to be retained and persist in medullary collecting ducts even after treatment is discontinued, suggesting a new mechanism of action for this drug. The purpose of the present study was to investigate whether Aliskiren regulates renal aquaporin expression in the collecting ducts and improves urinary concentrating defect induced by lithium in mice. The mice were fed with either normal chow or LiCl diet (40 mmol.kg dry food(-1).day(-1) for 4 days and 20 mmol.kg dry food(-1).day(-1) for the last 3 days) for 7 days. Some mice were intraperitoneally injected with Aliskiren (50 mg.kg body wt(-1).day(-1) in saline). Aliskiren significantly increased protein abundance of aquaporin-2 (AQP2) in the kidney inner medulla in mice. In inner medulla collecting duct cell suspension, Aliskiren markedly increased AQP2 and phosphorylated AQP2 at serine 256 (pS256-AQP2) protein abundance, which was significantly inhibited both by adenylyl cyclase inhibitor MDL-12330A and by PKA inhibitor H89, indicating an involvement of the cAMP-PKA signaling pathway in Aliskiren-induced increased AQP2 expression. Aliskiren treatment improved urinary concentrating defect in lithium-treated mice and partially prevented the decrease of AQP2 and pS256-AQP2 protein abundance in the inner medulla of the kidney. In conclusion, the direct renin inhibitor Aliskiren upregulates AQP2 protein expression in inner medullary collecting duct principal cells and prevents lithium-induced nephrogenic diabetes insipidus likely via cAMP-PKA pathways.


