SQ 22536Adenylyl cyclase inhibitor CAS# 17318-31-9 |
- D609
Catalog No.:BCC1509
CAS No.:83373-60-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 17318-31-9 | SDF | Download SDF |
| PubChem ID | 5270 | Appearance | Powder |
| Formula | C9H11N5O | M.Wt | 205.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (487.28 mM; Need ultrasonic) | ||
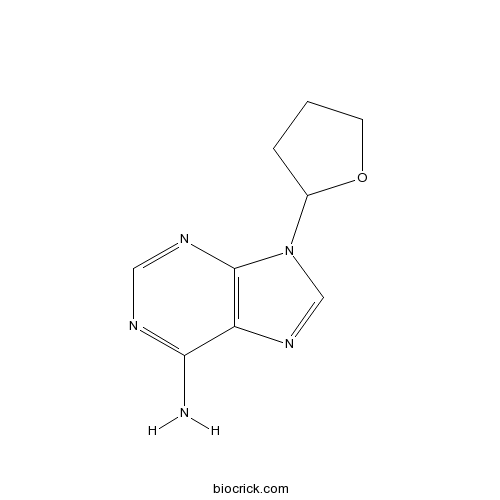
| Chemical Name | 9-(oxolan-2-yl)purin-6-amine | ||
| SMILES | C1CC(OC1)N2C=NC3=C2N=CN=C3N | ||
| Standard InChIKey | UKHMZCMKHPHFOT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H11N5O/c10-8-7-9(12-4-11-8)14(5-13-7)6-2-1-3-15-6/h4-6H,1-3H2,(H2,10,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of adenylyl cyclase (IC50 = 1.4 μM). Inhibits PGE1-stimulated increases in cAMP levels in intact human platelets. |

SQ 22536 Dilution Calculator

SQ 22536 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8728 mL | 24.3641 mL | 48.7282 mL | 97.4564 mL | 121.8205 mL |
| 5 mM | 0.9746 mL | 4.8728 mL | 9.7456 mL | 19.4913 mL | 24.3641 mL |
| 10 mM | 0.4873 mL | 2.4364 mL | 4.8728 mL | 9.7456 mL | 12.182 mL |
| 50 mM | 0.0975 mL | 0.4873 mL | 0.9746 mL | 1.9491 mL | 2.4364 mL |
| 100 mM | 0.0487 mL | 0.2436 mL | 0.4873 mL | 0.9746 mL | 1.2182 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SQ22536 is an effective adenylate cyclase (AC) inhibitor.
In Vitro:SQ22536 (SQ22,536) effectively inhibits the effect of forskolin with respective IC50 values of 5 μM.Preincubation with graded concentrations of SQ22536 reveals that both SQ22536 effectively inhibits PACAP-induced reporter gene activation with approximate IC50 value of 5 μM. SQ22536 more potently inhibits forskolin-induced Elk activation (IC50=10 μM) than 8-Br-cAMP-induced Elk activation (IC50=170 μM). Most notably, there are substantial differences in the reported potencies of SQ22536 to inhibit the activities of recombinant AC5 and AC6, with respective IC50 values of 2 μM and 360 μM. At a greater concentration (500 μM), SQ22536 significantly inhibits neurite elongation due to either forskolin or 8-Br-cAMP[1].
References:
[1]. Emery AC, et al. A new site and mechanism of action for the widely used adenylate cyclase inhibitor SQ22,536. Mol Pharmacol. 2013 Jan;83(1):95-105.
- Centaureidin
Catalog No.:BCN2575
CAS No.:17313-52-9
- Ganoderic acid DM
Catalog No.:BCN1113
CAS No.:173075-45-1
- Rhoifolin
Catalog No.:BCN1112
CAS No.:17306-46-6
- Rec 15/2615 dihydrochloride
Catalog No.:BCC7628
CAS No.:173059-17-1
- PMPA (NAALADase inhibitor)
Catalog No.:BCC2354
CAS No.:173039-10-6
- Goniothalamin
Catalog No.:BCN4690
CAS No.:17303-67-2
- Poloxime
Catalog No.:BCC5307
CAS No.:17302-61-3
- Isoanthricin
Catalog No.:BCN3531
CAS No.:17301-70-5
- BWX 46
Catalog No.:BCC5865
CAS No.:172997-92-1
- Biorobin
Catalog No.:BCN4691
CAS No.:17297-56-2
- FICZ
Catalog No.:BCC5513
CAS No.:172922-91-7
- PB 28 dihydrochloride
Catalog No.:BCC7411
CAS No.:172907-03-8
- 4-Beta-Hydroxycholesterol
Catalog No.:BCN2752
CAS No.:17320-10-4
- Clomipramine HCl
Catalog No.:BCC5036
CAS No.:17321-77-6
- Broussonetine A
Catalog No.:BCN2515
CAS No.:173220-07-0
- 2,5-Dihydroxy-1-methoxyxanthone
Catalog No.:BCN7577
CAS No.:173220-32-1
- TC-E 5003
Catalog No.:BCC8008
CAS No.:17328-16-4
- Garciniaxanthone E
Catalog No.:BCN1114
CAS No.:173294-74-1
- Isorhamnetin 3-glucoside-7-rhamnoside
Catalog No.:BCN1528
CAS No.:17331-71-4
- Aliskiren
Catalog No.:BCC1338
CAS No.:173334-57-1
- Aliskiren Hemifumarate
Catalog No.:BCC5018
CAS No.:173334-58-2
- Pelargonidin-3,5-O-diglucoside chloride
Catalog No.:BCN1527
CAS No.:17334-58-6
- Afobazole
Catalog No.:BCC5386
CAS No.:173352-21-1
- Rotundanonic acid
Catalog No.:BCN7152
CAS No.:173357-19-2
Inhibition of adenylate cyclase of catfish and rat hepatocyte membranes by 9-(tetrahydro-2-furyl)adenine (SQ 22536).[Pubmed:1669444]
J Enzyme Inhib. 1991;5(2):87-98.
The adenosine analogue 9-(Tetrahydro-2-furyl)adenine, SQ 22536, inhibited adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] activity of crude membrane preparations from catfish (Ictalurus melas) and rat isolated hepatocytes in a non-competitive manner. The IC50s were reduced in the presence of NaF. SQ 22536 reduced the activity of adenylate cyclase also in the presence of increasing concentrations of GTP, as well as Mg++ and Mn++. In the presence of catecholamines (epinephrine, norepinephrine, isoproterenol, phenylephrine) SQ 22536 reduced their activating effect on adenylate cyclase in both catfish and rat membranes. SQ 22536 also inhibited the effect of glucagon (0.1 microM) on rat membrane cyclase activity.
Effects of SQ 22536, an adenylyl cyclase inhibitor, on isoproterenol-induced cyclic AMP elevation and relaxation in newborn ovine pulmonary veins.[Pubmed:11858802]
Eur J Pharmacol. 2002 Feb 2;436(3):227-33.
The effects of inhibition of adenylyl cyclase on isoproterenol-induced relaxation were determined in isolated pulmonary veins of newborn lambs (7-12 days old). In veins constricted with endothelin-1, isoproterenol at concentrations < or = 3 x 10(-9) M had no effect on the cyclic AMP (cAMP) content but caused up to 56% relaxation. At higher concentrations (> or = 10(-8) M), isoproterenol elevated cAMP content and caused further relaxation. In veins constricted with endothelin-1 or U46619 (9,11-dideoxy-11, 9-epoxymethanoprostaglandin prostaglandin F2alpha), the cAMP elevation but not relaxation caused by isoproterenol was abolished by SQ 22536 [9-(tetrahydro-2-furanyl)-9H-purin-6-amine; an adenylyl cyclase inhibitor]. The effects of isoproterenol on vessel tension and cAMP content were inhibited by propranolol. Rp-8-CPT-cAMPS [8-(4-Chlorophenylthio)-adenosine-3',5'-cyclic monophosphorothioate, Rp-isomer] and Rp-8-Br-PET-cGMPS [beta-phenyl-1, N2-etheno-8-bromoguanosine-3',5'-cyclic monophosphorothioate, Rp-isomer], inhibitors of cAMP- and guanosine-3',5'-cyclic monophosphate (cGMP)-dependent protein kinases, respectively, attenuated relaxation caused by a cAMP analog but not that by isoproterenol. In the crude membrane preparations of pulmonary veins, an increase in the activity of adenylyl cyclase caused by isoproterenol was abolished by propranolol and SQ 22536. These results suggest that cAMP may not play a critical role in isoproterenol-induced relaxation of pulmonary veins of newborn lambs.
SQ 22536, an adenylate-cyclase inhibitor, prevents the antiplatelet effect of dazoxiben, a thromboxane-synthetase inhibitor.[Pubmed:6326343]
Thromb Haemost. 1984 Feb 28;51(1):125-8.
This study shows that dazoxiben, a selective inhibitor of thromboxane A2-synthetase in human platelets, inhibited arachidonic acid-induced platelet aggregation in platelet-rich plasma samples from four out of 16 healthy volunteers. In these four "responder" samples, the anti-aggregating effect of dazoxiben was prevented by the compound SQ 22536, a 9-substituted adenine analogue, endowed with an inhibitory activity on adenylate-cyclase. The compound SQ 22536 also counteracted the antiaggregating effect of prostaglandin D2, a known activator of platelet adenylate-cyclase. When platelet thromboxane A2-synthetase was blocked by dazoxiben, a marked increase of prostaglandin D2 was concomitantly observed both in "responder" and "non responder" samples. The compound SQ 22536 blunted the increase in platelet cAMP caused by either dazoxiben and sodium arachidonate or prostaglandin D2. It is suggested that the antiaggregating effect of dazoxiben is mediated by newly synthesized prostaglandin D2. The latter acts by stimulating adenylate-cyclase and increasing cAMP levels. The compound SQ 22536 prevents both phenomena. In "non responder" samples some factors--still to be defined--might counteract similarly to the compound SQ 22536 the antiaggregating activity of PGD2.
Role of cyclic nucleotides in vasodilations of the rat thoracic aorta induced by adenosine analogues.[Pubmed:11454656]
Br J Pharmacol. 2001 Jul;133(6):833-40.
Although adenosine analogues such as 5'-N-ethylcarboxamidoadenosine (NECA) relax the rat thoracic aorta in a partially endothelium-dependent manner via adenosine A(2A) receptors, others such as N(6)-R-phenylisopropyladenosine (R-PIA) act via an endothelium-independent, antagonist-insensitive mechanism. The role of cyclic nucleotides in these relaxations was investigated in isolated aortic rings using inhibitors of adenylate and guanylate cyclases as well as subtype-selective phosphodiesterase inhibitors. The adenylate cyclase inhibitor 9-(tetrahydro-2-furanyl)-9H-purin-6-amine (SQ 22536; 100 microM) significantly inhibited responses to NECA, but not responses to R-PIA. The type IV (cyclic AMP-selective) phosphodiesterase inhibitor 4-[(3-butoxy-4-methoxyphenyl)methyl]-2-imidazolidinone (RO 20-1724; 30 microM) significantly enhanced responses to NECA and to a lesser extent those to R-PIA. The guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3a]quinoxalin-1-one (ODQ; 100 microM) significantly inhibited responses to NECA and acetylcholine but not responses to R-PIA. The selective phosphodiesterase V (cyclic GMP-selective) inhibitors, zaprinast (10 microM) and 4-[[3',4'-(methylenedioxy)benzyl]amino]-6-methoxyquinazoline (MMQ; 1 microM), had no significant effect on responses to either NECA or R-PIA, but enhanced responses to acetylcholine. These results are consistent with the effects of NECA being via activation of endothelial receptors to release NO which stimulates guanylate cyclase, as well as smooth muscle receptors coupled to stimulation of adenylate cyclase. The lack of effect of zaprinast and MMQ on responses to NECA are likely to be due to simultaneous activation of both adenylate and guanylate cyclases in the smooth muscle, as cyclic AMP reduces the sensitivity of phosphodiesterase V to inhibitors. These results also suggest that the effects of R-PIA are via neither of these mechanisms.
Inhibition of adenylate cyclase in human blood platelets by 9-substituted adenine derivatives.[Pubmed:221552]
J Cyclic Nucleotide Res. 1979;5(2):125-34.
A series of 9-substituted adenine derivatives inhibited adenylate cyclase activity (ATP pyrophosphate-lyase (cyclizing) EC 4.6.1.1) of a particulate preparation of human blood platelets. A 3--6 fold elevation of adenylate cyclase activity by prostaglandin E1 (PGE1) was inhibited in a concentration-related manner by 9-(tetrahydro-5-methyl-2-furyl) adenine (SQ 22,538), 9-(tetrahydro-2-furyl) adenine (SQ 22,536), 9-cyclopentyladenine (SQ 22,534), 9-furfuryladenine (sQ 4647) and 9-benzyladenine (SQ 218611). The I50 values ranged from 21 microM for SQ 22,538 to 140 microM for SQ 21,611. These same adenine derivatives reversed the inhibition by PGE1 of ADP-induced aggregation and the PGE1-stimulated elevation of adenosine 3':5'-monophosphate (cyclic AMP). The reversal of platelet aggregation inhibition by SQ 22,536 and SQ 4647 was concentration-related with I50 values of 30 microM in each case, whereas SQ 22,534 and SQ 21,611 reversed inhibition by 30% at 100 microM. SQ 22,536, SQ 22,534 and SQ 21,611 also blocked the increase in cyclic AMP levels in a concentration-related manner with I50 values of 1, 4 and 60 microM, respectively. SQ 4647 inhibited the elevation of cyclic AMP by more than 85% at 1000 microM. The adenine derivatives had no effect on platelet aggregation or on cyclic AMP levels in the absence of PGE1. These results provide additional evidence that the inhibition of platelet aggregation by PGE1 is mediated by cyclic AMP.


