BGC 20-7615-HT6 antagonist,selective and high affinity CAS# 17375-63-2 |
- BEZ235 Tosylate
Catalog No.:BCC1416
CAS No.:1028385-32-1
- GSK2126458
Catalog No.:BCC3884
CAS No.:1086062-66-9
- VS-5584 (SB2343)
Catalog No.:BCC2047
CAS No.:1246560-33-7
- Everolimus (RAD001)
Catalog No.:BCC3594
CAS No.:159351-69-6
- PIK-294
Catalog No.:BCC4995
CAS No.:900185-02-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

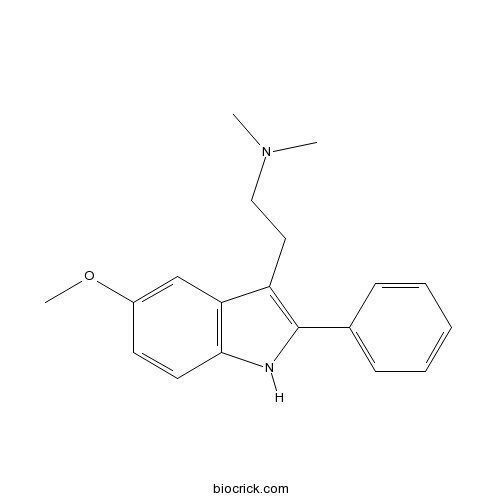
| Cas No. | 17375-63-2 | SDF | Download SDF |
| PubChem ID | 6918515 | Appearance | Powder |
| Formula | C19H22N2O | M.Wt | 294.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 50 mM in ethanol | ||
| Chemical Name | 2-(5-methoxy-2-phenyl-1H-indol-3-yl)-N,N-dimethylethanamine | ||
| SMILES | CN(C)CCC1=C(NC2=C1C=C(C=C2)OC)C3=CC=CC=C3 | ||
| Standard InChIKey | VSGPGYWZVPDDSK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H22N2O/c1-21(2)12-11-16-17-13-15(22-3)9-10-18(17)20-19(16)14-7-5-4-6-8-14/h4-10,13,20H,11-12H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective, high affinity 5-HT6 antagonist (Ki = 20 nM). Reverses the amnesic effects of scopolamine and enhances memory consolidation in a rat model. |

BGC 20-761 Dilution Calculator

BGC 20-761 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3969 mL | 16.9843 mL | 33.9685 mL | 67.9371 mL | 84.9214 mL |
| 5 mM | 0.6794 mL | 3.3969 mL | 6.7937 mL | 13.5874 mL | 16.9843 mL |
| 10 mM | 0.3397 mL | 1.6984 mL | 3.3969 mL | 6.7937 mL | 8.4921 mL |
| 50 mM | 0.0679 mL | 0.3397 mL | 0.6794 mL | 1.3587 mL | 1.6984 mL |
| 100 mM | 0.034 mL | 0.1698 mL | 0.3397 mL | 0.6794 mL | 0.8492 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BGC20-761 is a selective and high affinity antagonist of 5-HTC.
The 5-HT6 receptor, a G protein-coupled receptor (GPCR), is a subtype of 5-HT receptor which binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). This protein is expressed almost exclusively in the brain and mediates excitatory neurotransmission.
In cellular level, BGC20-761 (5-methoxy-2-phenyl-N,N-dimethyltryptamine) was shown to selectively blocked 5-HTC.
BGC20-761 was used to study the difference in drug- induced effects in memory consolidation in young and mature rats and human. In young mice, BGC20-761 treatment at doses of 5 mg/kg and 10 mg/kg i.p, dose-dependently reversed a deficit of social recognition induced by scopolamine, an anticholinergic drug that impairs memory at dosage of 0.4 mg/kg i.p. In mature rats (6 months), recognition of the novel object was improved following administration of BGC20-761. The difference in effects of BGC20-761 in young vs. mature rats may reflect the status of memory consolidation in these different age ranges 1.
Reference:
1. Mitchell ES, Hoplight BJ, Lear SP, et al. BGC20-761, a novel tryptamine analog, enhances memory consolidation and reverses scopolamine-induced memory deficit in social and visuospatial memory tasks through a 5-HT6 receptor-mediated mechanism. Neuropharmacology. 2006;50(4):412-420.
- TBB
Catalog No.:BCC1988
CAS No.:17374-26-4
- 3-Galloylquinic acid
Catalog No.:BCN3732
CAS No.:17365-11-6
- Desoxygambogenin
Catalog No.:BCN3068
CAS No.:173614-93-2
- 2,3-Didehydrosomnifericin
Catalog No.:BCN8005
CAS No.:173614-88-5
- Oxotremorine sesquifumarate
Catalog No.:BCC6814
CAS No.:17360-35-9
- 3,5-Dinitro-Tyr-OH
Catalog No.:BCC3331
CAS No.:17360-11-1
- Compound W
Catalog No.:BCC2341
CAS No.:173550-33-9
- Naphthoquine phosphate
Catalog No.:BCC1784
CAS No.:173531-58-3
- HMN-214
Catalog No.:BCC2517
CAS No.:173529-46-9
- Ficusin A
Catalog No.:BCN1115
CAS No.:173429-83-9
- Rotundanonic acid
Catalog No.:BCN7152
CAS No.:173357-19-2
- Afobazole
Catalog No.:BCC5386
CAS No.:173352-21-1
- Gambogin
Catalog No.:BCN3069
CAS No.:173792-67-1
- H-Gly-OBzl.HCl
Catalog No.:BCC2949
CAS No.:1738-68-7
- H-Ser-OBzl.HCl
Catalog No.:BCC3030
CAS No.:1738-72-3
- H-Gly-OBzl.TosOH
Catalog No.:BCC2948
CAS No.:1738-76-7
- H-Leu-OBzl.TosOH
Catalog No.:BCC2970
CAS No.:1738-77-8
- Swertiamarin
Catalog No.:BCN1116
CAS No.:17388-39-5
- Y-39983 dihydrochloride
Catalog No.:BCC4186
CAS No.:173897-44-4
- FR 171113
Catalog No.:BCC7734
CAS No.:173904-50-2
- Isocarapanaubine
Catalog No.:BCN1117
CAS No.:17391-09-2
- Corynoxine B
Catalog No.:BCN8454
CAS No.:17391-18-3
- Gambogenic acid
Catalog No.:BCN3077
CAS No.:173932-75-7
- Atrasentan
Catalog No.:BCC1379
CAS No.:173937-91-2
Elemene Induces Apoptosis of Human Gastric Cancer Cell Line BGC-823 via Extracellular Signal-Regulated Kinase (ERK) 1/2 Signaling Pathway.[Pubmed:28196062]
Med Sci Monit. 2017 Feb 14;23:809-817.
BACKGROUND Elemene is extracted from a traditional herbal medicine and is commonly used in the treatment of cancer in China. However, its effect on gastric cancer cells remains unknown. The goal of this study was to investigate its effect on human gastric cancer cells. MATERIAL AND METHODS Human gastric cancer BGC-823 cells and a tumor-bearing mouse model were employed to be divided into 4 groups: control group, elemene group, PD98059 group (an ERK 1/2 signaling pathway inhibitor), and the combined group (elemene plus PD98059). The tumor size, cell proliferation, expression of ERK 1/2 and phosphorylated ERK 1/2 (p-ERK 1/2), Bcl-2 mRNA, and Bax mRNA were measured. Moreover, cell apoptosis was detected and the apoptosis index was calculated. RESULTS Elemene and PD98059 each significantly inhibited the proliferation of gastric cancer cells BGC-823, and their combination showed higher synergistic inhibitory effect (P<0.05). We also found increased expression levels of p-ERK l/2 protein and Bax mRNA, but reduced level of Bcl-2 mRNA expression (P<0.05). Elemene presented higher apoptosis rate in a dose-dependent manner (P<0.05). Furthermore, the injection of elemene decreased the weight of transplanted tumors. CONCLUSIONS Elemene can inhibit the proliferation and induce the apoptosis of gastric cancer cells associated with the ERK 1/2 signaling pathway and expression levels of Bax mRNA and Bcl-2 mRNA.
Sulfamic and succinic acid derivatives of 25-OH-PPD and their activities to MCF-7, A-549, HCT-116, and BGC-823 cell lines.[Pubmed:28073676]
Bioorg Med Chem Lett. 2017 Feb 15;27(4):1076-1080.
In the search for new anti-tumor agents with higher potency than our previously identified compound 1 (25-OH-PPD, 25-hydroxyprotopanaxadiol), 12 novel sulfamic and succinic acid derivatives that could improve water solubility and contribute to good drug potency and pharmacokinetic profiles were designed and synthesized. Their in vitro anti-tumor activities in MCF-7, A-549, HCT-116, and BGC-823 cell lines and one normal cell line were tested by standard MTT assay. Results showed that compared with compound 1, compounds 2, 3, and 7 exhibited higher cytotoxic activity on A-549 and BGC-823 cell lines, together with lower toxicity in the normal cell. In particular, compound 2 exhibited the best anti-tumor activity in the in vitro assays, which may provide valuable data for the research and development of new anti-tumor agents.
Optimizing selective cutting strategies for maximum carbon stocks and yield of Moso bamboo forest using BIOME-BGC model.[Pubmed:28092748]
J Environ Manage. 2017 Apr 15;191:126-135.
The selective cutting method currently used in Moso bamboo forests has resulted in a reduction of stand productivity and carbon sequestration capacity. Given the time and labor expense involved in addressing this problem manually, simulation using an ecosystem model is the most suitable approach. The BIOME-BGC model was improved to suit managed Moso bamboo forests, which was adapted to include age structure, specific ecological processes and management measures of Moso bamboo forest. A field selective cutting experiment was done in nine plots with three cutting intensities (high-intensity, moderate-intensity and low-intensity) during 2010-2013, and biomass of these plots was measured for model validation. Then four selective cutting scenarios were simulated by the improved BIOME-BGC model to optimize the selective cutting timings, intervals, retained ages and intensities. The improved model matched the observed aboveground carbon density and yield of different plots, with a range of relative error from 9.83% to 15.74%. The results of different selective cutting scenarios suggested that the optimal selective cutting measure should be cutting 30% culms of age 6, 80% culms of age 7, and all culms thereafter (above age 8) in winter every other year. The vegetation carbon density and harvested carbon density of this selective cutting method can increase by 74.63% and 21.5%, respectively, compared with the current selective cutting measure. The optimized selective cutting measure developed in this study can significantly promote carbon density, yield, and carbon sink capacity in Moso bamboo forests.
Bioinformatic identification of candidate genes induced by trichostatin A in BGC-823 gastric cancer cells.[Pubmed:28356958]
Oncol Lett. 2017 Feb;13(2):777-783.
The aim of the present study was to identify the candidate genes induced by trichostatin A (TSA) in BGC-823 gastric cancer (GC) cells and to explore the possible inhibition mechanism of TSA in GC. Gene expression data were obtained through chip detection, and differentially expressed genes (DEGs) between GC cells treated with TSA and untreated GC cells (control group) were identified. Gene ontology analysis of the DEGs was performed using the database for annotation, visualization and integrated discovery. Then sub-pathway enrichment analysis was performed and a microRNA (miRNA) regulatory network was constructed. We selected 76 DEGs, among which 43 were downregulated genes and 33 were upregulated genes. By sub-pathway enrichment analysis of the DEGs, the propanoate metabolism pathway was selected as the sub-pathway. By constructing a miRNA regulatory network, we identified that DKK1 and KLF13 were the top hub nodes. The propanoate metabolism pathway and the genes DKK1 and KLF13 may play significant roles in the inhibition of GC induced by TSA. These genes may be potential therapeutic targets for GC. However, further experiments are still required to confirm our results.
BGC20-761, a novel tryptamine analog, enhances memory consolidation and reverses scopolamine-induced memory deficit in social and visuospatial memory tasks through a 5-HT6 receptor-mediated mechanism.[Pubmed:16298400]
Neuropharmacology. 2006 Mar;50(4):412-20.
Inhibition of 5-HT(6) receptors has been shown to improve memory consolidation, thus we tested whether a novel tryptamine analog with high affinity for 5-HT(6) receptors, BGC20-761 (5-methoxy-2-phenyl-N,N-dimethyltryptamine, PMDT), can enhance long-term memory. BGC20-761 (10 mg/kg i.p.) alone had no effect on social recognition in young rats, however, at doses of 5 mg/kg and 10 mg/kg i.p, BGC20-761 dose-dependently reversed a deficit of social recognition induced by scopolamine (0.4 mg/kg i.p.), an anticholinergic drug that impairs memory. BGC20-761 (10 mg/kg i.p.), scopolamine (0.2 mg/kg i.p.) or BGC20-761 + scopolamine had no effects on novel object discrimination in young rats (2 months). In mature rats (6 months), recognition of the novel object was improved following administration of BGC20-761. Scopolamine had no effect in object recognition. However, the addition of scopolamine disrupted the memory-enhancing effect of BGC20-761. Based on the high affinity of BGC20-761 for 5-HT(6) receptors, these cognitive enhancing effects are most likely mediated by 5-HT(6) receptor inhibition. The difference in effects of BGC20-761 in young vs. mature rats may reflect the status of memory consolidation in these different age ranges.
2-Substituted tryptamines: agents with selectivity for 5-HT(6) serotonin receptors.[Pubmed:10715164]
J Med Chem. 2000 Mar 9;43(5):1011-8.
Several 2-alkyl-5-methoxytryptamine analogues were designed and prepared as potential 5-HT(6) serotonin agonists. It was found that 5-HT(6) receptors accommodate small alkyl substituents at the indole 2-position and that the resulting compounds can bind with affinities comparable to that of serotonin. In particular, 2-ethyl-5-methoxy-N, N-dimethyltryptamine (8) binds with high affinity at human 5-HT(6) receptors (K(i) = 16 nM) relative to 5-HT (K(i) = 75 nM) and was a full agonist, at least as potent (8: K(act) = 3.6 nM) as serotonin (K(act) = 5.0 nM), in activating adenylate cyclase. Compound 8 displays modest affinity for several other populations of 5-HT receptors, notably h5-HT(1A) (K(i) = 170 nM), h5-HT(1D) (K(i) = 290 nM), and h5-HT(7) (K(i) = 300 nM) receptors, but is otherwise quite selective. Compound 8 represents the first and most selective 5-HT(6) agonist reported to date. Replacing the 2-ethyl substituent with a phenyl group results in a compound that retains 5-HT(6) receptor affinity (i.e., 10: K(i) = 20 nM) but lacks agonist character. 2-Substituted tryptamines, then, might allow entry to a novel class of 5-HT(6) agonists and antagonists.


