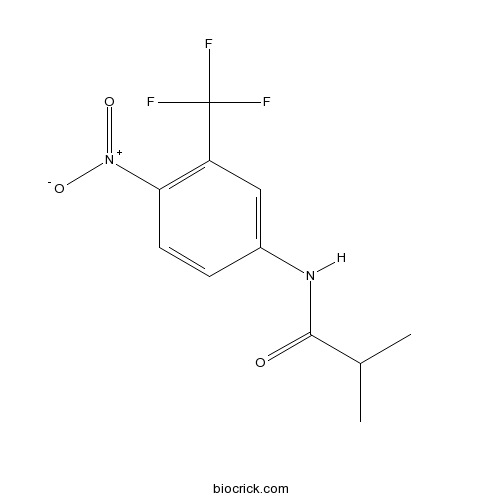
FlutamideNon-steroidal androgen receptor antagonist CAS# 13311-84-7 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13311-84-7 | SDF | Download SDF |
| PubChem ID | 3397 | Appearance | Powder |
| Formula | C11H11F3N2O3 | M.Wt | 276.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SCH 13521 | ||
| Solubility | DMSO : ≥ 100 mg/mL (362.04 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]propanamide | ||
| SMILES | CC(C)C(=O)NC1=CC(=C(C=C1)[N+](=O)[O-])C(F)(F)F | ||
| Standard InChIKey | MKXKFYHWDHIYRV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H11F3N2O3/c1-6(2)10(17)15-7-3-4-9(16(18)19)8(5-7)11(12,13)14/h3-6H,1-2H3,(H,15,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-steroidal antiandrogen; competitive antagonist of the androgen receptor. Blocks the biological activity of testosterone in vivo. |

Flutamide Dilution Calculator

Flutamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6204 mL | 18.1022 mL | 36.2043 mL | 72.4087 mL | 90.5108 mL |
| 5 mM | 0.7241 mL | 3.6204 mL | 7.2409 mL | 14.4817 mL | 18.1022 mL |
| 10 mM | 0.362 mL | 1.8102 mL | 3.6204 mL | 7.2409 mL | 9.0511 mL |
| 50 mM | 0.0724 mL | 0.362 mL | 0.7241 mL | 1.4482 mL | 1.8102 mL |
| 100 mM | 0.0362 mL | 0.181 mL | 0.362 mL | 0.7241 mL | 0.9051 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Flutamide is an antiandrogen drug, with its active metablolite binding at androgen receptor with Ki values of 55 nM, and primarily used to treat prostate cancer. Phase 4.
- Darifenacin HBr
Catalog No.:BCC4567
CAS No.:133099-07-7
- Darifenacin
Catalog No.:BCC1516
CAS No.:133099-04-4
- 3PO
Catalog No.:BCC5616
CAS No.:13309-08-5
- Crassifoline methine
Catalog No.:BCN1793
CAS No.:133084-00-1
- Fmoc-D-Lys(Boc)-OPfp
Catalog No.:BCC3527
CAS No.:133083-36-0
- BIM 23052
Catalog No.:BCC5945
CAS No.:133073-82-2
- ZD 7288
Catalog No.:BCC6884
CAS No.:133059-99-1
- Go 6983
Catalog No.:BCC3705
CAS No.:133053-19-7
- GF 109203X
Catalog No.:BCC3704
CAS No.:133052-90-1
- LY 225910
Catalog No.:BCC6891
CAS No.:133040-77-4
- Ethacrynic acid - d5
Catalog No.:BCC7987
CAS No.:1330052-59-9
- (+)-SK&F 10047 hydrochloride
Catalog No.:BCC6928
CAS No.:133005-41-1
- Epimedin B1
Catalog No.:BCN8199
CAS No.:133137-58-3
- GR 73632
Catalog No.:BCC5801
CAS No.:133156-06-6
- Oxypeucedan hydrate
Catalog No.:BCN8372
CAS No.:133164-11-1
- Fmoc-Cit-OH
Catalog No.:BCC3180
CAS No.:133174-15-9
- Fmoc-Thr(Trt)-OH
Catalog No.:BCC3554
CAS No.:133180-01-5
- PI3k-delta inhibitor 1
Catalog No.:BCC1861
CAS No.:1332075-63-4
- AM 92016 hydrochloride
Catalog No.:BCC6825
CAS No.:133229-11-5
- Heliangin
Catalog No.:BCN6487
CAS No.:13323-48-3
- 4-O-Methylbutein
Catalog No.:BCN6677
CAS No.:13323-67-6
- GSK2194069
Catalog No.:BCC8053
CAS No.:1332331-08-4
- KPT-185
Catalog No.:BCC4444
CAS No.:1333151-73-7
- TRPC6 inhibitor
Catalog No.:BCC4199
CAS No.:1333207-63-8
Flutamide-induced hypospadias in rats: A critical assessment.[Pubmed:28043016]
Differentiation. 2017 Mar - Apr;94:37-57.
This paper provides the first detailed description of Flutamide-induced hypospadias in the rat based upon wholemount, histologic, three-dimensional reconstruction, scanning electron microscopic, and immunocytochemical analysis. The penile malformations elicited by this potent anti-androgen include a substantial proximal shift in the urethral meatus that clearly conforms to the definition of hypospadias based upon specific morphological criteria for this malformation. Through examination of the normal penile development and Flutamide-induced abnormal penile development observed in prenatally oil- and Flutamide-treated rats, our analysis provides insights into the morphogenetic mechanism of development of hypospadias. In this regard, a common theme in normal penile development is midline fusion of epithelia followed by removal of the epithelial seam and establishment of midline mesenchymal confluence during development of the penile urethra and prepuce, processes which are impaired as a result of prenatal Flutamide treatment. The developmental processes occurring in normal penile development, through comparison with development of female external genitalia and those impaired due to prenatal Flutamide treatment, are consistent with critical role of androgen receptors in normal penile development in the rat, and the specific penile abnormalities embodied in Flutamide-induced rat hypospadias.
The impact of flutamide on prostaglandin F2alpha synthase and prostaglandin F2alpha receptor expression, and prostaglandin F2alpha concentration in the porcine corpus luteum of pregnancy.[Pubmed:28038404]
Domest Anim Endocrinol. 2017 Apr;59:81-89.
Recently, we have indicated that Flutamide-induced androgen deficiency diminished progesterone production in the porcine corpus luteum (CL) during late pregnancy and before parturition, as a sign of functional luteolysis. In pigs, the main luteolytic factor is prostaglandin F2alpha (PGF2alpha), which acts via specific receptors (PTGFRs), and its biosynthesis is catalyzed by prostaglandin F2alpha synthase (PGFS). The present study investigated the impact of Flutamide on luteal PGFS and PTGFR expression, as well as intraluteal PGF2alpha content during pregnancy in pigs. Flutamide (50 mg/kg BW per day, for 7 d) or corn oil (control groups) were administered subcutaneously into pregnant gilts (n = 3 per group) between 83 and 89 (GD90) or 101-107 (GD108) days of gestation (GD). On GD90 and GD108 ovaries were collected and CLs were obtained. Real-time PCR and Western blot analyses were conducted to quantify PGFS and PTGFR mRNA and protein expression, respectively. In addition, immunohistochemical localization of both proteins was performed and the concentration of PGF2alpha was analyzed by enzyme immunoassay method. Flutamide caused upregulation of PGFS mRNA and protein in GD90F (P = 0.008; P = 0.008, respectively) and GD108F (P = 0.041; P = 0.009, respectively) groups. The level of PTGFR mRNA increased only in the GD90F (P = 0.007) group, whereas PTGFR protein expression was greater in both gestational periods (P = 0.035; P = 0.038, respectively). On GD90 PGFS was immunolocalized in the cytoplasm of large luteal cells only, whereas on GD108, sparse small luteal cells also displayed positive staining. PTGFR showed membranous localization within large luteal cells on both days of pregnancy. In luteal tissue, PGF2alpha concentration was greater after Flutamide exposure on both days (P = 0.041; P = 0.038, respectively), when compared with control groups. Overall, the enhanced luteal PGF2alpha content due to increased PGFS expression after Flutamide administration might contribute to premature CL regression. Moreover, higher PTGFR protein levels indicate enhanced sensitivity of luteal cells to PGF2alpha under androgen deficiency.
Effects of Exposure to Estradiol Benzoate or Flutamide at the Weaning Age on Expression of Connexins in the Caudal Epididymis of Adult Rat.[Pubmed:28144639]
Dev Reprod. 2016 Dec;20(4):349-357.
The present research was chiefly designed to determine the effect of the treatment of estrogenic agonist, estradiol benzoate (EB), or antiandrogenic compound, Flutamide (Flu), at the weaning age on the expression of connexin (Cx) isoforms in the caudal epididymis of adult male rat. Animals were subcutaneously administrated with a single shot of either EB at a low-dose (0.015 microg of EB/kg body weight (BW)) or a high-dose (1.5 microg of EB/kg BW) or Flu at a low-dose (500 microg of EB/kg BW) or a high-dose (5 mg of EB/kg BW). Expressional changes of Cx isoforms in the adult caudal epididymis were examined by quantitative real-time PCR analysis. The treatment of a low-dose EB caused significant increases of Cx30.3, Cx31, Cx32, and Cx43 transcript levels but reduction of Cx31.1, Cx37, and Cx45 expression. Exposure to a high-dose EB resulted in very close responses observed in a low-dose EB treatment, except no significant expressional change of Cx37 and a significant induction of Cx40. Expression of all Cx isoforms, except Cx45, was significantly increased by a low-dose Flu treatment. Expressional increases of all Cx isoforms were detected by a high-dose Flu treatment. The current study demonstrates that a single exposure to estrogenic or antiandrogenic compound during the early postnatal developmental period is sufficient to disrupt normal expression of Cx isoforms in the adult caudal epididymis.
Diethylstilbestrol, flutamide and their combination impaired the spermatogenesis of male adult zebrafish through disrupting HPG axis, meiosis and apoptosis.[Pubmed:28213303]
Aquat Toxicol. 2017 Apr;185:129-137.
Both diethylstilbestrol (DES, an environmental estrogen) and Flutamide (FLU, an anti-androgen) are found to impair spermatogenesis by disrupting hypothalamic-pituitary-gonadal (HPG) axis and altering androgen levels through different mechanisms/modes of action in fish with poorly understood underlying mechanisms. Furthermore, it is not known whether and how a combined exposure of DES and FLU has a stronger effect than the compounds alone. In this study, male zebrafish adults were exposed to DES, FLU and their combination (DES+FLU) for 30days, and their effects on histological structure and sperm count in testis, androgen level in plasma, as well as the mRNA levels of genes involved in HPG axis, meiotic regulation and apoptosis were analyzed. After exposure, DES and FLU disrupted spermatogenesis in zebrafish, and their combination resulted in even more severe impairment, indicating the inhibitory roles of these chemicals on spermatogenesis and their additive effects on zebrafish. The different regulation of vtg1 expression in the liver in response to DES and FLU further confirmed the different modes of action of these drugs. Gene expression and plasma steroid level analyses demonstrated the suppressed mRNA levels of the key genes (such as gnrh3, fshbeta and lhbeta in brain and dmrt1, sf1, cyp17a1 and cyp11b2 in testis) in HPG axis and decreased 11-ketotestosterone (11-KT) levels in plasma. The declined level of 11-KT was thus supposed to be closely related to the down-regulation of cyp26a1 (encoding the catabolic enzyme of retinoic acid) and suppression of genes involved in meiotic regulation (nanos1, dmc1 and sycp3). In fish exposed to DES and DES+FLU, enhanced apoptosis (elevated bax/bcl-2 expression ratio) was also observed. The suppression of meiotic regulation in response to all the exposures and enhanced apoptosis in response to DES were thus supposed to result in the spermatogenic impairment in zebrafish. The present study greatly extends our understanding on the mechanisms underlying of reproductive toxicity of environment estrogens and anti-androgens in fish.
Drug safety is a barrier to the discovery and development of new androgen receptor antagonists.[Pubmed:20878947]
Prostate. 2011 Apr;71(5):480-8.
BACKGROUND: Androgen receptor (AR) antagonists are part of the standard of care for prostate cancer. Despite the almost inevitable development of resistance in prostate tumors to AR antagonists, no new AR antagonists have been approved for over a decade. Treatment failure is due in part to mutations that increase activity of AR in response to lower ligand concentrations as well as to mutations that result in AR response to a broader range of ligands. The failure to discover new AR antagonists has occurred in the face of continued research; to enable progress, a clear understanding of the reasons for failure is required. METHODS: Non-clinical drug safety studies and safety pharmacology assays were performed on previously approved AR antagonists (bicalutamide, Flutamide, nilutamide), next generation antagonists in clinical testing (MDV3100, BMS-641988), and a pre-clinical drug candidate (BMS-501949). In addition, non-clinical studies with AR mutant mice, and EEG recordings in rats were performed. Non-clinical findings are compared to disclosures of clinical trial results. RESULTS: As a drug class, AR antagonists cause seizure in animals by an off-target mechanism and are found in vitro to inhibit GABA-A currents. Clinical trials of candidate next generation AR antagonists identify seizure as a clinical safety risk. CONCLUSIONS: Non-clinical drug safety profiles of the AR antagonist drug class create a significant barrier to the identification of next generation AR antagonists. GABA-A inhibition is a common off-target activity of approved and next generation AR antagonists potentially explaining some side effects and safety hazards of this class of drugs.
Induction of human arylamine N-acetyltransferase type I by androgens in human prostate cancer cells.[Pubmed:17210686]
Cancer Res. 2007 Jan 1;67(1):85-92.
Human arylamine N-acetyltransferases (NAT) bioactivate arylamine and heterocyclic amine carcinogens present in red meat and tobacco products. As a result, factors that regulate expression of NATs have the potential to modulate cancer risk in individuals exposed to these classes of carcinogens. Because epidemiologic studies have implicated well-done meat consumption as a risk factor for prostate cancer, we have investigated the effects of androgens on the expression of arylamine N-acetyltransferase type I (NAT1). We show that NAT1 activity is induced by R1881 in androgen receptor (AR)-positive prostate lines 22Rv1 and LNCaP, but not in the AR-negative PC-3, HK-293, or HeLa cells. The effect of R1881 was dose dependent, with an EC(50) for R1881 of 1.6 nmol/L. Androgen up-regulation of NAT1 was prevented by the AR antagonist Flutamide. Real-time PCR showed a significant increase in NAT1 mRNA levels for R1881-treated cells (6.60 +/- 0.80) compared with vehicle-treated controls (1.53 +/- 0.17), which was not due to a change in mRNA stability. The increase in NAT1 mRNA was attenuated by concurrent cycloheximide treatment, suggesting that the effect of R1881 may not be by direct transcriptional activation of NAT1. The dominant NAT1 transcript present following androgen treatment was type IIA, indicating transcriptional activation from the major NAT1 promoter P1. A series of luciferase reporter deletions mapped the androgen responsive motifs to a 157-bp region of P1 located 745 bases upstream of the first exon. These results show that human NAT1 is induced by androgens, which may have implications for cancer risk in individuals.
Flutamide. Mechanism of action of a new nonsteroidal antiandrogen.[Pubmed:1270239]
Invest Urol. 1976 May;13(6):429-34.
Flutamide is a new nonsteroidal antiandrogen useful in the treatment of prostatic carcinoma. The mechanism of action of this compound was investigated using the sex accessory tissue of the male rat. Flutamide was found to block the action of both endogenous and exogenous testosterone and, in addition, to be a potent inhibitor of testosterone-stimulated prostatic DNA synthesis. Moreover, it is capable of inhibiting prostatic nuclear uptake of androgen. Electron microscopic observations further confirm the antiandrogenic properties of Flutamide.


