PseudobufarenoginCAS# 17008-69-4 |
- Bufarenogin
Catalog No.:BCN2297
CAS No.:17008-65-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 17008-69-4 | SDF | Download SDF |
| PubChem ID | 204810 | Appearance | Powder |
| Formula | C24H32O6 | M.Wt | 416.51 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Synonyms | ψ-Bufarenogin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-[(3S,5R,8R,9S,10S,12R,13S,14S,17R)-3,12,14-trihydroxy-10,13-dimethyl-11-oxo-2,3,4,5,6,7,8,9,12,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]pyran-2-one | ||
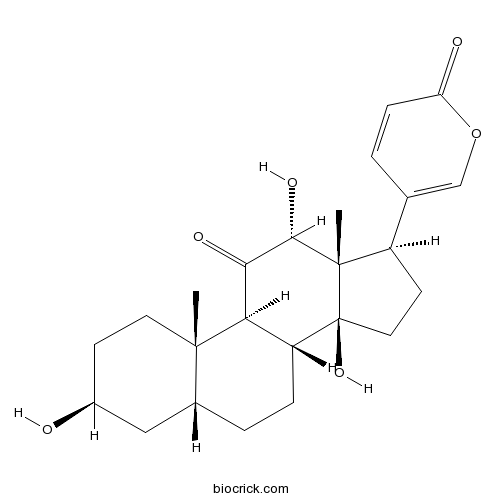
| SMILES | CC12CCC(CC1CCC3C2C(=O)C(C4(C3(CCC4C5=COC(=O)C=C5)O)C)O)O | ||
| Standard InChIKey | SOGONHOGEFLVPE-BHZHDSHXSA-N | ||
| Standard InChI | InChI=1S/C24H32O6/c1-22-9-7-15(25)11-14(22)4-5-17-19(22)20(27)21(28)23(2)16(8-10-24(17,23)29)13-3-6-18(26)30-12-13/h3,6,12,14-17,19,21,25,28-29H,4-5,7-11H2,1-2H3/t14-,15+,16-,17-,19-,21+,22+,23+,24+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pseudobufarenogin(ψ-bufarenogin), a novel anti-tumor compound, suppresses liver cancer growth by inhibiting receptor tyrosine kinase-mediated signaling. 2. ψ-Bufarenogin shows inhibition of human kidney Na(+)/K(+)-ATPase activity. |
| Targets | Sodium Channel | ATPase | Potassium Channel | MEK | ERK | PI3K | Akt | EGFR | Raf |

Pseudobufarenogin Dilution Calculator

Pseudobufarenogin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4009 mL | 12.0045 mL | 24.009 mL | 48.0181 mL | 60.0226 mL |
| 5 mM | 0.4802 mL | 2.4009 mL | 4.8018 mL | 9.6036 mL | 12.0045 mL |
| 10 mM | 0.2401 mL | 1.2005 mL | 2.4009 mL | 4.8018 mL | 6.0023 mL |
| 50 mM | 0.048 mL | 0.2401 mL | 0.4802 mL | 0.9604 mL | 1.2005 mL |
| 100 mM | 0.024 mL | 0.12 mL | 0.2401 mL | 0.4802 mL | 0.6002 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pseudobufarenogin is a natural compound extracted from toad species with unknown details.
References:
[1]. Jin Ding, et al. ψ-Bufarenogin, a novel anti-tumor compound, suppresses liver cancer growth by inhibiting receptor tyrosine kinase-mediated signaling. Oncotarget. 2015 May 10; 6(13): 11627–11639.
- Bufarenogin
Catalog No.:BCN2297
CAS No.:17008-65-0
- Reserpine hydrochloride
Catalog No.:BCC4279
CAS No.:16994-56-2
- LY 333531 hydrochloride
Catalog No.:BCC7969
CAS No.:169939-93-9
- Iso-cuparenal
Catalog No.:BCN7350
CAS No.:16982-01-7
- Mesuol
Catalog No.:BCN6583
CAS No.:16981-20-7
- Dibutyryl-cAMP, sodium salt
Catalog No.:BCC8079
CAS No.:16980-89-5
- Angelol K
Catalog No.:BCN8142
CAS No.:169736-93-0
- Ro 60-0175 fumarate
Catalog No.:BCC7196
CAS No.:169675-09-6
- Z-Thr(tBu)-OH.DCHA
Catalog No.:BCC2565
CAS No.:16966-07-7
- Isodonal
Catalog No.:BCN3390
CAS No.:16964-56-0
- Trichosanatine
Catalog No.:BCN1818
CAS No.:169626-16-8
- Odoratone
Catalog No.:BCN1105
CAS No.:16962-90-6
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- Curcolone
Catalog No.:BCN3559
CAS No.:17015-43-9
- Nepicastat (SYN-117) HCl
Catalog No.:BCC2286
CAS No.:170151-24-3
- 11-Keto-beta-boswellic acid
Catalog No.:BCN2298
CAS No.:17019-92-0
- Bauerenol acetate
Catalog No.:BCN1106
CAS No.:17020-04-1
- 24-Methylenecycloartane-3beta,26-diol
Catalog No.:BCN1530
CAS No.:17020-27-8
- CD 2314
Catalog No.:BCC6071
CAS No.:170355-37-0
- CD 2665
Catalog No.:BCC7778
CAS No.:170355-78-9
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- 4-(6-Methyl-4-oxohept-5-en-2-yl)cyclohex-2-en-1-one
Catalog No.:BCN7528
CAS No.:170380-68-4
- 1,4-Epidioxybisabola-2,10-dien-9-one
Catalog No.:BCN7532
CAS No.:170380-69-5
- Isohyperectine
Catalog No.:BCN3405
CAS No.:170384-75-5
Bufadienolides from parotoid gland secretions of Cuban toad Peltophryne fustiger (Bufonidae): Inhibition of human kidney Na(+)/K(+)-ATPase activity.[Pubmed:26615828]
Toxicon. 2016 Feb;110:27-34.
Parotoid gland secretions of toad species are a vast reservoir of bioactive molecules with a wide range of biological properties. Herein, for the first time, it is described the isolation by preparative reversed-phase HPLC and the structure elucidation by NMR spectroscopy and/or mass spectrometry of nine major bufadienolides from parotoid gland secretions of the Cuban endemic toad Peltophryne fustiger: psi-bufarenogin, gamabufotalin, bufarenogin, arenobufagin, 3-(N-suberoylargininyl) marinobufagin, bufotalinin, telocinobufagin, marinobufagin and bufalin. In addition, the secretion was analyzed by UPLC-MS/MS which also allowed the identification of azelayl arginine. The effect of arenobufagin, bufalin and psi-bufarenogin on Na(+)/K(+)-ATPase activity in a human kidney preparation was evaluated. These bufadienolides fully inhibited the Na(+)/K(+)-ATPase in a concentration-dependent manner, although arenobufagin (IC50 = 28.3 nM) and bufalin (IC50 = 28.7 nM) were 100 times more potent than psi-bufarenogin (IC50 = 3020 nM). These results provided evidence about the importance of the hydroxylation at position C-14 in the bufadienolide skeleton for the inhibitory activity on the Na(+)/K(+)-ATPase.
psi-Bufarenogin, a novel anti-tumor compound, suppresses liver cancer growth by inhibiting receptor tyrosine kinase-mediated signaling.[Pubmed:25890498]
Oncotarget. 2015 May 10;6(13):11627-39.
Resistance of hepatocellular carcinoma (HCC) to existing chemotherapeutic agents largely contributes to the poor prognosis of patients, and discovery of novel anti-HCC drug is in an urgent need. Herein we report psi-Bufarenogin, a novel active compound that we isolated from the extract of toad skin, exhibited potent therapeutic effect in xenografted human hepatoma without notable side effects. In vitro, psi-Bufarenogin suppressed HCC cells proliferation through impeding cell cycle progression, and it facilitated cell apoptosis by downregulating Mcl-1 expression. Moreover, psi-Bufarenogin decreased the number of hepatoma stem cells through Sox2 depression and exhibited synergistic effect with conventional chemotherapeutics. Mechanistic study revealed that psi-Bufarenogin impaired the activation of MEK/ERK pathway, which is essential in the proliferation of hepatoma cells. psi-Bufarenogin notably suppressed PI3-K/Akt cascade, which was required in psi-Bufarenogin-mediated reduction of Mcl-1 and Sox2. psi-Bufarenogin inhibited the auto-phosphorylation and activation of epithelial growth factor receptor (EGFR) and hepatocyte growth factor receptor (c-Met), thereafter suppressed their primary downstream cascades Raf/MEK/ERK and PI3-K/Akt signaling. Taken together, psi-Bufarenogin suppressed HCC growth via inhibiting, at least partially, receptor tyrosine kinases-regulated signaling, suggesting that psi-Bufarenogin could be a novel lead compound for anti-HCC drug.


