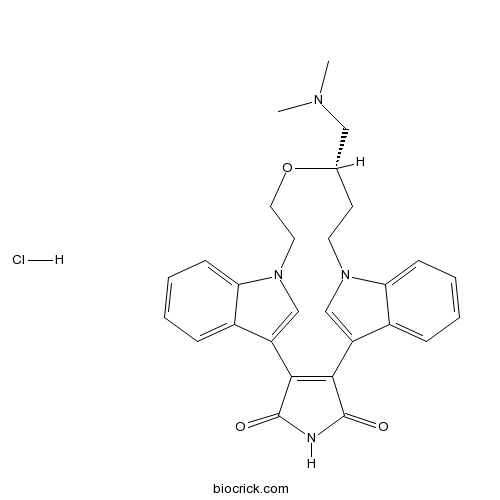
LY 333531 hydrochlorideCAS# 169939-93-9 |
- WYE-354
Catalog No.:BCC1059
CAS No.:1062169-56-5
- GDC-mTOR inhibitor
Catalog No.:BCC1781
CAS No.:1207358-59-5
- GDC-0349
Catalog No.:BCC1094
CAS No.:1207360-89-1
- QL-IX-55
Catalog No.:BCC1876
CAS No.:1223002-54-7
- Nordihydroguaiaretic acid
Catalog No.:BCC1805
CAS No.:500-38-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 169939-93-9 | SDF | Download SDF |
| PubChem ID | 9870785 | Appearance | Powder |
| Formula | C28H29ClN4O3 | M.Wt | 505.01 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LY 333531 hydrochloride | ||
| Solubility | DMSO : 6.67 mg/mL (13.21 mM; Need ultrasonic) | ||
| SMILES | CN(C)CC1CCN2C=C(C3=CC=CC=C32)C4=C(C5=CN(CCO1)C6=CC=CC=C65)C(=O)NC4=O.Cl | ||
| Standard InChIKey | NYQIEYDJYFVLPO-FERBBOLQSA-N | ||
| Standard InChI | InChI=1S/C28H28N4O3.ClH/c1-30(2)15-18-11-12-31-16-21(19-7-3-5-9-23(19)31)25-26(28(34)29-27(25)33)22-17-32(13-14-35-18)24-10-6-4-8-20(22)24;/h3-10,16-18H,11-15H2,1-2H3,(H,29,33,34);1H/t18-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Isozyme-selective inhibitor of protein kinase C (PKC); competitively and reversibly inhibits PKCβI and PKCβII (IC50 values are 4.7 and 5.9 nM respectively). Selective for PKCβ over other PKC isozymes (IC50 values are 0.052, 0.25, 0.30, 0.36, 0.60 and >100 μM for PKCη, -δ, -γ, -α, -ε and -ζ respectively). Exhibits selectivity for PKC over other ATP-dependent kinases, including protein kinase A, casein kinase and src). |

LY 333531 hydrochloride Dilution Calculator

LY 333531 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9802 mL | 9.9008 mL | 19.8016 mL | 39.6032 mL | 49.504 mL |
| 5 mM | 0.396 mL | 1.9802 mL | 3.9603 mL | 7.9206 mL | 9.9008 mL |
| 10 mM | 0.198 mL | 0.9901 mL | 1.9802 mL | 3.9603 mL | 4.9504 mL |
| 50 mM | 0.0396 mL | 0.198 mL | 0.396 mL | 0.7921 mL | 0.9901 mL |
| 100 mM | 0.0198 mL | 0.099 mL | 0.198 mL | 0.396 mL | 0.495 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ruboxistaurin hydrochloride is a selective and ATP-competitive PKCβ inhibitor, with IC50s of 4.7 and 5.9 nM for PKCβI and PKCβII, shows less potent inhibition on PKCη (IC50, 52 nM), PKCα (IC50, 360 nM), PKCγ (IC50, 300 nM), PKCδ (IC50, 250 nM), and has no effect on PKCζ (IC50, >100 μM).
In Vitro:Ruboxistaurin hydrochloride is a selective and ATP-competitive PKCβ inhibitor, with IC50s of 4.7 and 5.9 nM for PKCβI and PKCβII, shows less potent inhibition on PKCη (IC50, 52 nM), PKCα (IC50, 360 nM), PKCγ (IC50, 300 nM), PKCδ (IC50, 250 nM), and has no effect on PKCζ (IC50, >100 μM)[1]. Ruboxistaurin (10 and 400 nM) dramatically inhibits glucose-induced monocyte adherence to levels that are not different from baseline adherence of monocytes to endothelial cells under NG conditions. Ruboxistaurin (10 and 400 nM) dose not alter the endothelial expression of adhesion molecules or modify endothelial cell growth[2]. Ruboxistaurin (LY333531; 10 nM) reduces high-glucose (HG)-induced human renal glomerular endothelial cells (HRGECs) viability, and inhibits the increases in swiprosin-1 in HRGECs incubated with HG[3].
In Vivo:Ruboxistaurin (LY333531; 1 mg/kg/d for 8 weeks) markedly reduces GEC apoptosis as well as swiprosin-1 upregulation, and ameliorates renal glomerular injury in the diabetic mice. Ruboxistaurin also potently attenuates the expression of PARP, cleaved-caspase9, cleaved-caspase3, and the Bax/Bcl-2 ratio, in diabetic mice[3]. Ruboxistaurin (LY333531; 0.1, 1.0, or 10.0 mg/kg/d, po.o.) dramatically reduces the number of leukocytes trapped in the retinal microcirculation of diabetic rats[4].
References:
[1]. Jirousek MR, et al. (S)-13-[(dimethylamino)methyl]-10,11,14,15-tetrahydro-4,9:16, 21-dimetheno-1H, 13H-dibenzo[e,k]pyrrolo[3,4-h][1,4,13]oxadiazacyclohexadecene-1,3(2H)-d ione (LY333531) and related analogues: isozyme selective inhibitors of protein kinase C beta. J Med Chem. 1996 Jul 5;39(14):2664-71.
[2]. Kunt T, et al. The beta-specific protein kinase C inhibitor ruboxistaurin (LY333531) suppresses glucose-induced adhesion of human monocytes to endothelial cells in vitro. J Diabetes Sci Technol. 2007 Nov;1(6):929-35.
[3]. Wang ZB, et al. LY333531, a PKCβ inhibitor, attenuates glomerular endothelial cell apoptosis in the early stage of mouse diabetic nephropathy via down-regulating swiprosin-1. Acta Pharmacol Sin. 2017 Jul;38(7):1009-1023.
[4]. Nonaka A, et al. PKC-beta inhibitor (LY333531) attenuates leukocyte entrapment in retinal microcirculation of diabetic rats. Invest Ophthalmol Vis Sci. 2000 Aug;41(9):2702-6.
- Iso-cuparenal
Catalog No.:BCN7350
CAS No.:16982-01-7
- Mesuol
Catalog No.:BCN6583
CAS No.:16981-20-7
- Dibutyryl-cAMP, sodium salt
Catalog No.:BCC8079
CAS No.:16980-89-5
- Angelol K
Catalog No.:BCN8142
CAS No.:169736-93-0
- Ro 60-0175 fumarate
Catalog No.:BCC7196
CAS No.:169675-09-6
- Z-Thr(tBu)-OH.DCHA
Catalog No.:BCC2565
CAS No.:16966-07-7
- Isodonal
Catalog No.:BCN3390
CAS No.:16964-56-0
- Trichosanatine
Catalog No.:BCN1818
CAS No.:169626-16-8
- Odoratone
Catalog No.:BCN1105
CAS No.:16962-90-6
- Celecoxib
Catalog No.:BCC1099
CAS No.:169590-42-5
- Deracoxib
Catalog No.:BCC4108
CAS No.:169590-41-4
- Floricaline
Catalog No.:BCN2104
CAS No.:16958-32-0
- Reserpine hydrochloride
Catalog No.:BCC4279
CAS No.:16994-56-2
- Bufarenogin
Catalog No.:BCN2297
CAS No.:17008-65-0
- Pseudobufarenogin
Catalog No.:BCN8234
CAS No.:17008-69-4
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- Curcolone
Catalog No.:BCN3559
CAS No.:17015-43-9
- Nepicastat (SYN-117) HCl
Catalog No.:BCC2286
CAS No.:170151-24-3
- 11-Keto-beta-boswellic acid
Catalog No.:BCN2298
CAS No.:17019-92-0
- Bauerenol acetate
Catalog No.:BCN1106
CAS No.:17020-04-1
- 24-Methylenecycloartane-3beta,26-diol
Catalog No.:BCN1530
CAS No.:17020-27-8
- CD 2314
Catalog No.:BCC6071
CAS No.:170355-37-0
- CD 2665
Catalog No.:BCC7778
CAS No.:170355-78-9
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5


