SC 236Selective cyclooxygenase-2 (COX-2) inhibitor CAS# 170569-86-5 |
- Verteporfin
Catalog No.:BCC3690
CAS No.:129497-78-5
- Methylcobalamin
Catalog No.:BCC5188
CAS No.:13422-55-4
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- Miglustat
Catalog No.:BCC5187
CAS No.:72599-27-0
- Grape Seed Extract
Catalog No.:BCC5317
CAS No.:84929-27-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 170569-86-5 | SDF | Download SDF |
| PubChem ID | 9865808 | Appearance | Powder |
| Formula | C16H11ClF3N3O2S | M.Wt | 401.79 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SC 58236 | ||
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
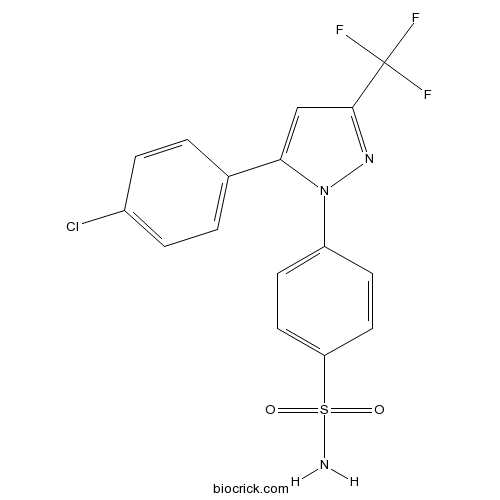
| Chemical Name | 4-[5-(4-chlorophenyl)-3-(trifluoromethyl)pyrazol-1-yl]benzenesulfonamide | ||
| SMILES | C1=CC(=CC=C1C2=CC(=NN2C3=CC=C(C=C3)S(=O)(=O)N)C(F)(F)F)Cl | ||
| Standard InChIKey | NSQNZEUFHPTJME-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H11ClF3N3O2S/c17-11-3-1-10(2-4-11)14-9-15(16(18,19)20)22-23(14)12-5-7-13(8-6-12)26(21,24)25/h1-9H,(H2,21,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective COX-2 inhibitor (IC50 values are 0.005 and 17.8 μM for COX-2 and COX-1 respectively). Displays anti-inflammatory properties and potent antimetastatic activity against both experimental metastases and spontaneous metastases arising following primary tumor excision. |

SC 236 Dilution Calculator

SC 236 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4889 mL | 12.4443 mL | 24.8886 mL | 49.7772 mL | 62.2216 mL |
| 5 mM | 0.4978 mL | 2.4889 mL | 4.9777 mL | 9.9554 mL | 12.4443 mL |
| 10 mM | 0.2489 mL | 1.2444 mL | 2.4889 mL | 4.9777 mL | 6.2222 mL |
| 50 mM | 0.0498 mL | 0.2489 mL | 0.4978 mL | 0.9955 mL | 1.2444 mL |
| 100 mM | 0.0249 mL | 0.1244 mL | 0.2489 mL | 0.4978 mL | 0.6222 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isohyperectine
Catalog No.:BCN3405
CAS No.:170384-75-5
- 1,4-Epidioxybisabola-2,10-dien-9-one
Catalog No.:BCN7532
CAS No.:170380-69-5
- 4-(6-Methyl-4-oxohept-5-en-2-yl)cyclohex-2-en-1-one
Catalog No.:BCN7528
CAS No.:170380-68-4
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- CD 2665
Catalog No.:BCC7778
CAS No.:170355-78-9
- CD 2314
Catalog No.:BCC6071
CAS No.:170355-37-0
- 24-Methylenecycloartane-3beta,26-diol
Catalog No.:BCN1530
CAS No.:17020-27-8
- Bauerenol acetate
Catalog No.:BCN1106
CAS No.:17020-04-1
- 11-Keto-beta-boswellic acid
Catalog No.:BCN2298
CAS No.:17019-92-0
- Nepicastat (SYN-117) HCl
Catalog No.:BCC2286
CAS No.:170151-24-3
- Curcolone
Catalog No.:BCN3559
CAS No.:17015-43-9
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- Oteromycin
Catalog No.:BCN1849
CAS No.:170591-45-4
- YC 1
Catalog No.:BCC7912
CAS No.:170632-47-0
- Fmoc-D-Abu-OH
Catalog No.:BCC3203
CAS No.:170642-27-0
- α-Conotoxin EI
Catalog No.:BCC5979
CAS No.:170663-33-9
- D-Mannitol diacetonide
Catalog No.:BCC8951
CAS No.:1707-77-3
- Bindone
Catalog No.:BCC8877
CAS No.:1707-95-5
- 6beta-Hydroxyhispanone
Catalog No.:BCN7453
CAS No.:170711-93-0
- Nociceptin
Catalog No.:BCC5686
CAS No.:170713-75-4
- 11-Deoxymogroside V
Catalog No.:BCN8143
CAS No.:1707161-17-8
- Aprepitant
Catalog No.:BCC1101
CAS No.:170729-80-3
- Trityl candesartan cilexetil
Catalog No.:BCC9188
CAS No.:170791-09-0
- Astressin
Catalog No.:BCC5790
CAS No.:170809-51-5
The COX-2 inhibitor SC-236 exerts anti-inflammatory effects by suppressing phosphorylation of ERK in a murine model.[Pubmed:17822719]
Life Sci. 2007 Aug 23;81(11):863-72.
SC-236, (4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1-pyrazol-1-]benzenesulfonamide; C(16)H(11)ClF(3)N(3)O(2)S) is a highly selective cyclooxygenase (COX)-2 inhibitor. Recently, there have been reports that SC-236 protects against cartilage damage in addition to reducing inflammation and pain for those with osteoarthritis. However, the mechanism involved in an inflammatory allergic reaction in a murine model has not been examined. The aim of the present study is to elucidate whether and how SC-236 modulates the inflammatory allergic reaction in a murine model. In this study, the anti-allergic effect was investigated using rat peritoneal mast cells, IgE-induced passive cutaneous anaphylaxis (PCA), and the ear-swelling model in mice. Also, we examined the inhibitory effect of SC-236 on the expression of interleukin (IL)-6 and tumor necrosis factor (TNF)-alpha. SC-236 was found to inhibit the ear-swelling response and histamine release in the murine model. Additionally, SC-236 was revealed to inhibit the PCA response and COX-2 expression. As a final step, the inhibitory mechanism of SC-236 was shown to occur through phosphorylation of extracellular signal-regulated protein kinase (ERK). These in vitro and in vivo results provide new insight into the pharmacological actions of SC-236 as a potential molecule for therapy for inflammatory allergic diseases.
The selective cyclooxygenase-2 inhibitor SC-236 reduces liver fibrosis by mechanisms involving non-parenchymal cell apoptosis and PPARgamma activation.[Pubmed:15876570]
FASEB J. 2005 Jul;19(9):1120-2.
The importance of inflammation in initiating the sequence of events that lead to liver fibrosis is increasingly recognized. In this study, we tested the effects of SC-236, a selective cyclooxygenase (COX)-2 inhibitor, in rats with carbon tetrachloride (CCl4)-induced liver fibrosis. Livers from CCl4-treated rats showed increased COX-2 expression and 15-deoxy-prostaglandin (PG)J2 (15d-PGJ2) formation, as well as decreased peroxisome proliferator-activated receptor (PPAR)gamma expression. In these animals, SC-236 reduced liver fibrosis as revealed by histological analysis and by a reduction in hepatic hydroxyproline levels, metalloproteinase-2 activity, and alpha-smooth muscle actin expression. Interestingly, SC-236 normalized 15d-PGJ2 levels and restored PPARgamma expression in the liver of CCl4-treated rats. In isolated hepatic stellate cells (HSCs)--the major player in liver fibrogenesis--and Kupffer cells--the cell type primarily responsible for increased hepatic COX-2-SC-236 exhibited remarkable pro-apoptotic and growth inhibitory properties. Of interest, SC-236 decreased HSC viability to a similar extent than the PPARgamma ligand rosiglitazone. Moreover, SC-236 significantly induced PPARgamma expression in HSCs and acted as a potent PPARgamma agonist in a luciferase-reporter trans-activation assay. These data indicate that, by mechanisms involving non-parenchymal cell apoptosis and PPARgamma activation, the selective COX-2 inhibitor SC-236 might have therapeutic potential for prevention of liver fibrosis.
The regulatory effect of SC-236 (4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1-pyrazol-1-l]benzenesulfonamide) on stem cell factor induced migration of mast cells.[Pubmed:17320132]
Toxicol Appl Pharmacol. 2007 Apr 15;220(2):138-45.
SC-236, (4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1-pyrazol-1-]benzenesulfonamide; C(16)H(11)ClF(3)N(3)O(2)S), is a highly selective cyclooxygenase (COX)-2 inhibitor. Recently, there have been reports that SC-236 protects against cartilage damage in addition to reducing inflammation and pain in osteoarthritis. However, the mechanism involved in the inflammatory allergic reaction has not been examined. Mast cells accumulation can be related to inflammatory conditions, including allergic rhinitis, asthma, and rheumatoid arthritis. The aim of the present study is to investigate the effects of SC-236 on stem cell factor (SCF)-induced migration, morphological alteration, and cytokine production of rat peritoneal mast cells (RPMCs). We observed that SCF significantly induced the migration and morphological alteration. The ability of SCF to enhance migration and morphological alteration was abolished by treatment with SC-236. In addition, production of tumor necrosis factor (TNF)-alpha, interleukin (IL)-1beta, and vascular endothelial growth factor (VEGF) production induced by SCF was significantly inhibited by treatment with SC-236. Previous work has demonstrated that SCF-induced migration and cytokine production of mast cells require p38 MAPK activation. We also showed that SC-236 suppresses the SCF-induced p38 MAPK activation in RPMCs. These data suggest that SC-236 inhibits migration and cytokine production through suppression of p38 MAPK activation. These results provided new insight into the pharmacological actions of SC-236 and its potential therapeutic role in the treatment of inflammatory allergic diseases.
Cyclooxygenase-2 inhibitor SC-236 [4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1-pyrazol-1-l] benzenesulfonamide] suppresses nuclear factor-kappaB activation and phosphorylation of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and c-Jun N-terminal kinase in human mast cell line cells.[Pubmed:15784648]
J Pharmacol Exp Ther. 2005 Jul;314(1):27-34.
SC-236 [4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1-pyrazol-1-l] benzenesulfonamide; C16H11ClF3N3O2S] is a highly selective cyclooxygenase (COX)-2 inhibitor. However, the exact mechanism that accounts for the anti-inflammatory effect of SC-236 is not completely understood. The aim of the present study was to elucidate whether and how SC-236 modulates the inflammatory reaction in a stimulated human mast cell (HMC) line, HMC-1. SC-236 inhibited the expression of tumor necrosis factor-alpha, interleukin (IL)-6, IL-8, vascular endothelial growth factor, COX-2, inducible nitric-oxide synthase, and hypoxia-inducible factor-1alpha in phorbol 12-myristate 13-acetate plus calcium ionophore A23187 (PMACI)-stimulated HMC-1. SC-236 suppressed nuclear factor (NF)-kappaB activation induced by PMACI, leading to suppression of IkappaB-alpha phosphorylation and degradation. SC-236 also suppressed strong induction of NF-kappaB promoter-mediated luciferase activity. In addition, SC-236 suppressed PMACI-induced phosphorylation of the mitogen-activated protein kinase p38, the extracellular-regulated kinase p44, and the c-Jun N-terminal kinase and induced expression of mitogen-activated protein kinase phosphatase-1. These results provide new insight into the pharmacological actions of SC-236 as a potential molecule for therapy of mast cell-mediated inflammatory diseases.
Antimetastatic activity of a cyclooxygenase-2 inhibitor.[Pubmed:15213717]
Br J Cancer. 2004 Jul 19;91(2):359-65.
Cyclooxygenase-2 (COX-2) expression is increased in breast cancer and surgery has been shown to increase the growth of metastatic tumours. We investigated the effect of selective COX-2 inhibition on the growth of metastases in either an experimental metastasis model or following excision of a murine primary breast tumour. 50,000 4T1 mammary carcinoma cells were injected into the mammary fat pad of female BALB/c mice. When the mean TD reached 8+/-0.4 mm, tumours were excised and the mice were randomised into two groups (n=12 per group) to receive daily intraperitoneal injections of the selective COX-2 inhibitor, SC-236 or drug vehicle for 14 days. Alternatively, experimental metastases were established by tail-vein injection of 50,000 4T1 cells. Mice received either the selective COX-2 inhibitor, SC-236 or drug vehicle for 14 days (n=12 per group). SC-236 treatment significantly reduced tumour burden, the number and size of spontaneous metastases following primary tumour excision. SC-236 treatment also reduced tumour burden, the number and size of experimental metastases. Immunohistochemical staining demonstrated that COX-2 inhibition reduced microvessel density and increased apoptosis within both spontaneous and experimental metastases. These data clearly demonstrate that the selective COX-2 inhibitor, SC-236, has potent antimetastatic activity against both spontaneous metastases arising following primary tumour excision and experimental metastases.
Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib).[Pubmed:9135032]
J Med Chem. 1997 Apr 25;40(9):1347-65.
A series of sulfonamide-containing 1,5-diarylpyrazole derivatives were prepared and evaluated for their ability to block cyclooxygenase-2 (COX-2) in vitro and in vivo. Extensive structure-activity relationship (SAR) work was carried out within this series, and a number of potent and selective inhibitors of COX-2 were identified. Since an early structural lead (1f, SC-236) exhibited an unacceptably long plasma half-life, a number of pyrazole analogs containing potential metabolic sites were evaluated further in vivo in an effort to identify compounds with acceptable pharmacokinetic profiles. This work led to the identification of 1i (4-[5-(4-methylphenyl)-3-(trifluoromethyl)- H-pyrazol-1-yl]benzenesulfonamide, SC-58635, celecoxib), which is currently in phase III clinical trials for the treatment of rheumatoid arthritis and osteoarthritis.
A single amino acid difference between cyclooxygenase-1 (COX-1) and -2 (COX-2) reverses the selectivity of COX-2 specific inhibitors.[Pubmed:8663121]
J Biol Chem. 1996 Jun 28;271(26):15810-4.
Nonsteroidal anti-inflammatory drugs (NSAIDs) currently available for clinical use inhibit both COX-1 and COX-2. This suggests that clinically useful NSAIDs inhibit pro-inflammatory prostaglandins (PGs) derived from the activity of COX-2, as well as PGs in tissues like the stomach and kidney (via COX-1). A new class of compounds has recently been developed (SC-58125) that have a high degree of selectivity for the inducible form of cyxlooxygenase (COX-2) over the constitutive form (COX-1). This unique class of compounds exhibit a time-dependent irreversible inhibition of COX-2, while reversibly inhibiting COX-1. The molecular basis of this selectivity was probed by site-directed mutagenesis of the active site of COX-2. The sequence differences in the active site were determined by amino acid replacement of the COX-2 sequences based on the known crystal structure of COX-1, which revealed a single amino acid difference in the active site (valine 509 to isoleucine) and a series of differences at the mouth of the active site. Mutants with the single amino acid substitution in the active site and a combination of three changes in the mouth of the active site were made in human COX-2, expressed in insect cells and purified. The single amino acid change of valine 509 to isoleucine confers selectivity of COX-2 inhibitors in the class of SC-58125 and others of the same class (SC-236, NS-398), while commonly used NSAIDs such as indomethacin showed no change in selectivity. Substitutions of COX-1 sequences in COX-2 at the mouth of the active site of COX-2 did not change the selectivity of SC-58125. This indicates that the single amino acid substitution of isoleucine at position 509 for a valine is sufficient to confer COX-2 selectivity in this example of a diaryl-heterocycle COX inhibitor.


