PashanoneCAS# 42438-78-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 42438-78-8 | SDF | Download SDF |
| PubChem ID | 6254251 | Appearance | Yellow cryst. |
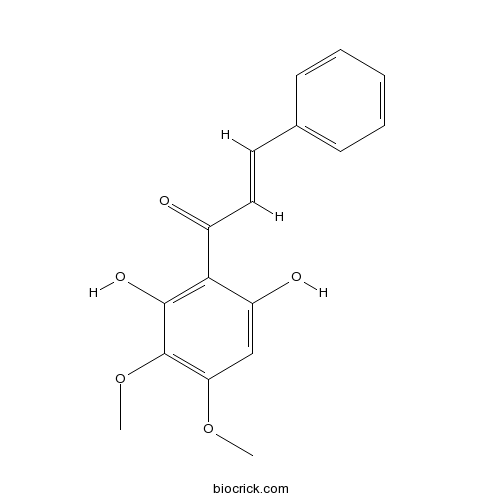
| Formula | C17H16O5 | M.Wt | 300.3 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-1-(2,6-dihydroxy-3,4-dimethoxyphenyl)-3-phenylprop-2-en-1-one | ||
| SMILES | COC1=C(C(=C(C(=C1)O)C(=O)C=CC2=CC=CC=C2)O)OC | ||
| Standard InChIKey | KVDNSLPRNTZIKF-CMDGGOBGSA-N | ||
| Standard InChI | InChI=1S/C17H16O5/c1-21-14-10-13(19)15(16(20)17(14)22-2)12(18)9-8-11-6-4-3-5-7-11/h3-10,19-20H,1-2H3/b9-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pashanone possesses moderate antifungal activity. 2. Pashanone exhibits cytotoxic activity. |
| Targets | Antifection |

Pashanone Dilution Calculator

Pashanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.33 mL | 16.65 mL | 33.3 mL | 66.6001 mL | 83.2501 mL |
| 5 mM | 0.666 mL | 3.33 mL | 6.66 mL | 13.32 mL | 16.65 mL |
| 10 mM | 0.333 mL | 1.665 mL | 3.33 mL | 6.66 mL | 8.325 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.665 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-Benzylimidazole
Catalog No.:BCC8462
CAS No.:4238-71-5
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
- Buxtamine
Catalog No.:BCC8135
CAS No.:4236-73-1
- (-)-Gallocatechin gallate
Catalog No.:BCN6328
CAS No.:4233-96-9
- SC 57461A
Catalog No.:BCC2348
CAS No.:423169-68-0
- PYZD-4409
Catalog No.:BCC4253
CAS No.:423148-78-1
- N-(4-Cyanophenyl)glycine
Catalog No.:BCC9057
CAS No.:42288-26-6
- Hesperadin
Catalog No.:BCC2174
CAS No.:422513-13-1
- Triacetyl Resveratrol
Catalog No.:BCC6482
CAS No.:42206-94-0
- DOI hydrochloride
Catalog No.:BCC5925
CAS No.:42203-78-1
- Cephalocyclidin A
Catalog No.:BCN5481
CAS No.:421583-14-4
- Artemyriantholide D
Catalog No.:BCN7478
CAS No.:421558-76-1
- Pinostilbene
Catalog No.:BCN5483
CAS No.:42438-89-1
- H-ß-Ala-OEt.HCl
Catalog No.:BCC2852
CAS No.:4244-84-2
- Flunixin Meglumin
Catalog No.:BCC4429
CAS No.:42461-84-7
- Isobutyl 4-Hydroxybenzoate
Catalog No.:BCN8412
CAS No.:4247-02-3
- 23-Hydroxylongispinogenin
Catalog No.:BCN7830
CAS No.:42483-24-9
- IPA-3
Catalog No.:BCC4978
CAS No.:42521-82-4
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- Crocin
Catalog No.:BCN2373
CAS No.:42553-65-1
- Sotrastaurin (AEB071)
Catalog No.:BCC3857
CAS No.:425637-18-9
- 8-Hydroxy-5-O-beta-D-glucopyranosylpsoralen
Catalog No.:BCN1443
CAS No.:425680-98-4
- Luteolin-6-C-glucoside
Catalog No.:BCN4985
CAS No.:4261-42-1
- TAK-700 (Orteronel)
Catalog No.:BCC2280
CAS No.:426219-18-3
An unusual homoisoflavanone and a structurally-related dihydrochalcone from Polygonum ferrugineum (Polygonaceae).[Pubmed:16884749]
Phytochemistry. 2006 Oct;67(19):2152-8.
The homoisoflavanone 5,7-dihydroxy-6-methoxy-3-(9-hydroxy-phenylmethyl)-chroman-4-one (1) and its structurally related 2',4',6'-trihydroxy-3'-methoxy-alpha-hydroxymethyl-beta-hydroxy-dihydrochalcone (2) along with the known Pashanone (3), flavokawin B (4) and cardamonin or alpinetin chalcone (5) pinostrobin (6) and 5,8-dimethoxy-7-hydroxychroman-4-one (7) were isolated from dry leaves of Polygonum ferrugineum (Polygonaceae). To our knowledge, this is the first report of the isolation of a homoisoflavanone from the Polygonum genus and the Polygonaceae family, and could be an important chemotaxonomic finding. In addition, the pattern of substitution of this homoisoflavanone is different from others previously reported.
A new hydrochalcone from Miliusa sinensis.[Pubmed:21859261]
Nat Prod Res. 2011 Aug;25(14):1361-5.
A new dihydrochalcone 4',6'-dihydroxy-2',3',4-trimethoxydihydrochalcone (1) along with nine known compounds, Pashanone (2), dihydroPashanone (3), pinostrobin (4), 5-hydroxy-7,4'-dimethoxyflavanone (5), 5-hydroxy-6,7-dimethoxyflavanone (6), 5-hydroxy-7,8-dimethoxyflavanone (7), 24-methylencycloartane-3beta,21-diol (8), liriodenine (9) and 3,5-dihydroxy-7,3',4'-trimethoxyflavone (10), were isolated from the extracts, exhibiting cytotoxic activity (n-hexane and ethyl acetate extracts) of Miliusa sinensis. The structure of (1) was elucidated by the analysis of spectral data (IR, HR-MS, EI-MS, 1D and 2D NMR).
Detection of antifungal compounds in Polygonum ferrugineum Wedd. extracts by bioassay-guided fractionation. Some evidences of their mode of action.[Pubmed:22001591]
J Ethnopharmacol. 2011 Nov 18;138(2):633-6.
ETHNOPHARMACOLOGICAL RELEVANCE: Polygonum ferrugineum Wedd. (Polygonaceae) is used to heal infected wounds and as antiseptic, antibiotic or antifungal in the traditional Argentinean medicine. The present investigation was carried out to evaluate the antifungal properties of different extracts of aerial parts of Polygonum ferrugineum, in order to give support to its ethnopharmacological use and to isolate the compounds responsible for the antifungal properties. The most active compounds were tested for their capacity of producing hyphae malformations, similar to those previously observed for crude extracts. MATERIALS AND METHODS: Agar Dilution Method (ADM) and Agar Overlay Bioautography (AOB) were used for bioassay-guided fractionation of the aerial part extracts against a panel of human opportunistic pathogenic fungi. The Neurospora crassa assay, followed by Optical Microscopy and Scanning Electron Microscopy observation, was used for studies of mechanisms of action. RESULTS: MeOH extract and DCM and Hex sub-extracts, but not Aq, EtOAc or BuOH ones possess antifungal activity. Of the seven isolated compounds, cardamonin 2 showed a selective inhibition of Epidermophyton floccosum with a very low MIC (=6.2 mug/mL) and Pashanone 1 possessed moderate antifungal activity (MICs=25-50 mug/mL) but a broader spectrum of action. Chalcone 2, but not 1, induced swelling and shortening of the Neurospora crassa hyphae, similar as those caused by the crude DCM extract. CONCLUSIONS: The bioassay-guided fractionation of Polygonum ferrugineum DCM extract allowed the isolation of five active compounds. Among them, cardamonin 2 showed the highest antifungal activity and selectivity towards Epidermophyton floccosum; in addition, it induced Neurospora crassa malformations that are similar than those produced by the crude DCM extract. These results give additional support to the ethnopharmacological use of Polygonum ferrugineum as antifungal agent.


