Triacetyl ResveratrolCell-permeable resveratrol prodrug CAS# 42206-94-0 |
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 42206-94-0 | SDF | Download SDF |
| PubChem ID | 5962587 | Appearance | Powder |
| Formula | C20H18O6 | M.Wt | 354.35 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | >13.85mg/mL in DMSO | ||
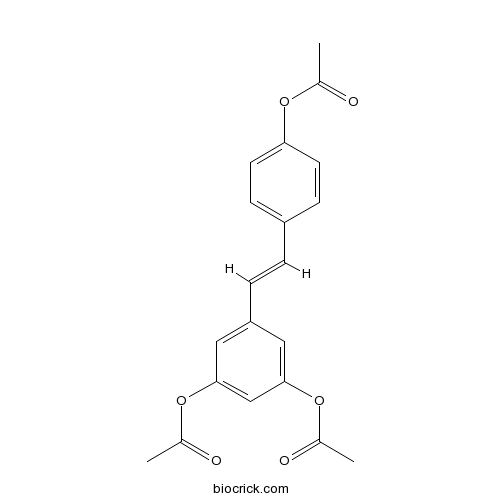
| Chemical Name | [4-[(E)-2-(3,5-diacetyloxyphenyl)ethenyl]phenyl] acetate | ||
| SMILES | CC(=O)OC1=CC=C(C=C1)C=CC2=CC(=CC(=C2)OC(=O)C)OC(=O)C | ||
| Standard InChIKey | PDAYUJSOJIMKIS-SNAWJCMRSA-N | ||
| Standard InChI | InChI=1S/C20H18O6/c1-13(21)24-18-8-6-16(7-9-18)4-5-17-10-19(25-14(2)22)12-20(11-17)26-15(3)23/h4-12H,1-3H3/b5-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Triacetylresveratrol has anti-cancer activity,it inhibits the phosphorylation of STAT3 and NFκB, down-regulates Mcl-1, and up-regulates Bim and Puma in pancreatic cancer cells. |
| Targets | STAT | NF-kB | Mcl-1 | Bim | Puma |
| In vitro | In vitro comparative studies of resveratrol and triacetylresveratrol on cell proliferation, apoptosis, and STAT3 and NFκB signaling in pancreatic cancer cells.[Pubmed: 27539371]Sci Rep. 2016 Aug 19;6:31672.Resveratrol (RES) has been studied extensively as an anticancer agent. However, the anticancer effects of Triacetylresveratrol (TRES, an acetylated analog of RES) which has higher bioavailability have not been well established. Novel chemical library screen identifies naturally occurring plant products that specifically disrupt glioblastoma-endothelial cell interactions.[Pubmed: 26286961]Oncotarget. 2015 Jul 30;6(21):18282-92.Tumor growth is not solely a consequence of autonomous tumor cell properties. Rather, tumor cells act upon and are acted upon by their microenvironment. It is tumor tissue biology that ultimately determines tumor growth. Thus, we developed a compound library screen for agents that could block essential tumor-promoting effects of the glioblastoma (GBM) perivascular stem cell niche (PVN). We modeled the PVN with three-dimensional primary cultures of human brain microvascular endothelial cells in Matrigel. We previously demonstrated stimulated growth of GBM cells in this PVN model and used this to assay PVN function. |

Triacetyl Resveratrol Dilution Calculator

Triacetyl Resveratrol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8221 mL | 14.1103 mL | 28.2207 mL | 56.4414 mL | 70.5517 mL |
| 5 mM | 0.5644 mL | 2.8221 mL | 5.6441 mL | 11.2883 mL | 14.1103 mL |
| 10 mM | 0.2822 mL | 1.411 mL | 2.8221 mL | 5.6441 mL | 7.0552 mL |
| 50 mM | 0.0564 mL | 0.2822 mL | 0.5644 mL | 1.1288 mL | 1.411 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5644 mL | 0.7055 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Triacetyl Resveratrol is a cell-permeable and more stable resveratrol prodrug [1].
Triacetyl Resveratrol is a potential and cell-permeable resveratrol prodrug. In 32D-cl3 cells, triacetyl resveratrol (3', 5',4'-Tri-O-acetylresveratrol) protected cells from irradiation and increased radioresistance, and is slightly more potent than resveratrol [1]. In lysates derived from HEK293-TYR cells, triacetyl resveratrol inhibited human TYR activity with IC50 value of 20 μM. In HEMs, triacetyl resveratrol was less cytotoxic than resveratrol. In both murine melanoma B16/F10 cells and HEMs, triacetyl resveratrol effectively inhibited intracellular melanin contents increased by α-MSH and L-tyrosine, respectively. Triacetyl Resveratrol was easily digested to resveratrol by esterases [2]. In LNCaP cells, triacetyl-resveratrol activated p53, increased p21 and p53R2 and decreased PSA expression. In CWR22Rv1 cells (mutated p53), triacetyl-resveratrol induced G1/S arrest [3]. In MCF-7 and MDA-MB-231 breast cancer cells, triacetyl-resveratrol interacted avidly and specifically with integrin αvβ3 through binding at the site targeted by the RGD peptide. Triacetyl-resveratrol induced ERK and p38 phosphorylation [4].
In γ-irradiated mice, 3,5,4'-Tri-O-acetylresveratrol (10 mg/kg) prior to γ-irradiation exhibited significant protective activity with 80% survival rate. Moreover, 3', 5',4'-Tri-O-acetylresveratrol had longer half-life that may assist more rapid distribution to tissues [1].
References:
[1]. Koide K, Osman S, Garner AL, et al. The Use of 3,5,4'-Tri-O-acetylresveratrol as a Potential Pro-drug for Resveratrol Protects Mice from γ-Irradiation-Induced Death. ACS Med Chem Lett, 2011, 2(4): 270-274.
[2]. Park J, Park JH, Suh HJ, et al. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch Dermatol Res, 2014, 306(5): 475-487.
[3]. Hsieh TC, Huang YC, Wu JM. Control of prostate cell growth, DNA damage and repair and gene expression by resveratrol analogues, in vitro. Carcinogenesis, 2011, 32(1): 93-101.
[4]. Hsieh TC, Wong C, John Bennett D, et al. Regulation of p53 and cell proliferation by resveratrol and its derivatives in breast cancer cells: an in silico and biochemical approach targeting integrin αvβ3. Int J Cancer, 2011, 129(11): 2732-2743.
- DOI hydrochloride
Catalog No.:BCC5925
CAS No.:42203-78-1
- Cephalocyclidin A
Catalog No.:BCN5481
CAS No.:421583-14-4
- Artemyriantholide D
Catalog No.:BCN7478
CAS No.:421558-76-1
- N,N-Bis(2-chloroethyl)-p-toluenesulphonamide
Catalog No.:BCC9060
CAS No.:42137-88-2
- Tetradehydropodophyllotoxin
Catalog No.:BCN8395
CAS No.:42123-27-3
- H2L 5765834
Catalog No.:BCC6311
CAS No.:420841-84-5
- AK-7
Catalog No.:BCC5426
CAS No.:420831-40-9
- Lucialdehyde A
Catalog No.:BCN2449
CAS No.:420781-84-6
- 2 CTC
Catalog No.:BCC2571
CAS No.:42074-68-0
- d[Leu4,Lys8]-VP
Catalog No.:BCC5981
CAS No.:42061-33-6
- Atractylenolide III acetate
Catalog No.:BCC9147
CAS No.:
- Mezlocillin Sodium
Catalog No.:BCC4678
CAS No.:42057-22-7
- Hesperadin
Catalog No.:BCC2174
CAS No.:422513-13-1
- N-(4-Cyanophenyl)glycine
Catalog No.:BCC9057
CAS No.:42288-26-6
- PYZD-4409
Catalog No.:BCC4253
CAS No.:423148-78-1
- SC 57461A
Catalog No.:BCC2348
CAS No.:423169-68-0
- (-)-Gallocatechin gallate
Catalog No.:BCN6328
CAS No.:4233-96-9
- Buxtamine
Catalog No.:BCC8135
CAS No.:4236-73-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
- 1-Benzylimidazole
Catalog No.:BCC8462
CAS No.:4238-71-5
- Pashanone
Catalog No.:BCN5482
CAS No.:42438-78-8
- Pinostilbene
Catalog No.:BCN5483
CAS No.:42438-89-1
- H-ß-Ala-OEt.HCl
Catalog No.:BCC2852
CAS No.:4244-84-2
- Flunixin Meglumin
Catalog No.:BCC4429
CAS No.:42461-84-7
Novel chemical library screen identifies naturally occurring plant products that specifically disrupt glioblastoma-endothelial cell interactions.[Pubmed:26286961]
Oncotarget. 2015 Jul 30;6(21):18282-92.
Tumor growth is not solely a consequence of autonomous tumor cell properties. Rather, tumor cells act upon and are acted upon by their microenvironment. It is tumor tissue biology that ultimately determines tumor growth. Thus, we developed a compound library screen for agents that could block essential tumor-promoting effects of the glioblastoma (GBM) perivascular stem cell niche (PVN). We modeled the PVN with three-dimensional primary cultures of human brain microvascular endothelial cells in Matrigel. We previously demonstrated stimulated growth of GBM cells in this PVN model and used this to assay PVN function. We screened the Microsource Spectrum Collection library for drugs that specifically blocked PVN function, without any direct effect on GBM cells themselves. Three candidate PVN-disrupting agents, Iridin, Tigogenin and Triacetylresveratrol (TAR), were identified and evaluated in secondary in vitro screens against a panel of primary GBM isolates as well as in two different in vivo intracranial models. Iridin and TAR significantly inhibited intracranial tumor growth and prolonged survival in these mouse models. Together these data identify Iridin and TAR as drugs with novel GBM tissue disrupting effects and validate the importance of preclinical screens designed to address tumor tissue function rather than the mechanisms of autonomous tumor cell growth.
In vitro comparative studies of resveratrol and triacetylresveratrol on cell proliferation, apoptosis, and STAT3 and NFkappaB signaling in pancreatic cancer cells.[Pubmed:27539371]
Sci Rep. 2016 Aug 19;6:31672.
Resveratrol (RES) has been studied extensively as an anticancer agent. However, the anticancer effects of triacetylresveratrol (TRES, an acetylated analog of RES) which has higher bioavailability have not been well established. We comparatively evaluated their effects on cell proliferation, apoptosis and the molecular changes in STAT3, NFkappaB and apoptotic signaling pathways in pancreatic cancer cells. Apoptosis was determined by flow cytometry. The nuclear translocation and interaction of STAT3 and NFkappaB were detected by Western blotting and immunoprecipitation, respectively. Both TRES and RES inhibited cell viability, and induced apoptosis of pancreatic cancer cells in a concentration and incubation time-dependent manner. TRES, similarly to RES, inhibited the phosphorylation of STAT3 and NFkappaB, down-regulated Mcl-1, and up-regulated Bim and Puma in pancreatic cancer cells. Remarkably, we, for the first time, observed that both TRES and RES suppressed the nuclear translocation, and interrupted the interaction of STAT3 and NFkappaB in PANC-1 cells. Comparative anticancer effects of TRES and RES on pancreatic cancer suggested that TRES with higher bioavailability may be a potential agent for pancreatic cancer prevention and treatment. Further in vivo experiments and functional studies are warranted to investigate whether TRES exhibits better beneficial effects than RES in mice and humans.


