AmrubicinTopoisomerase II inhibitor, anthracycline agent CAS# 110267-81-7 |
- Arctiin
Catalog No.:BCN1090
CAS No.:20362-31-6
- Daunorubicin HCl
Catalog No.:BCC5083
CAS No.:23541-50-6
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Flumequine
Catalog No.:BCC5090
CAS No.:42835-25-6
- Amonafide
Catalog No.:BCC1249
CAS No.:69408-81-7
- Fostriecin sodium salt
Catalog No.:BCC2460
CAS No.:87860-39-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 110267-81-7 | SDF | Download SDF |
| PubChem ID | 178149 | Appearance | Powder |
| Formula | C25H25NO9 | M.Wt | 483.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AMR | ||
| Solubility | >16.45mg/mL in DMSO | ||
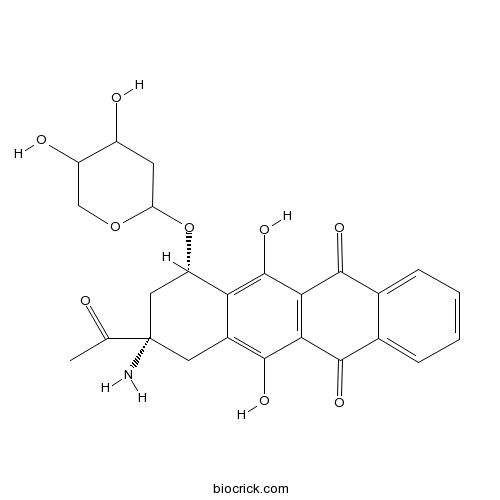
| Chemical Name | (7S,9S)-9-acetyl-9-amino-7-(4,5-dihydroxyoxan-2-yl)oxy-6,11-dihydroxy-8,10-dihydro-7H-tetracene-5,12-dione | ||
| SMILES | CC(=O)C1(CC(C2=C(C3=C(C(=C2C1)O)C(=O)C4=CC=CC=C4C3=O)O)OC5CC(C(CO5)O)O)N | ||
| Standard InChIKey | VJZITPJGSQKZMX-HUVCIAIMSA-N | ||
| Standard InChI | InChI=1S/C25H25NO9/c1-10(27)25(26)7-13-18(16(8-25)35-17-6-14(28)15(29)9-34-17)24(33)20-19(23(13)32)21(30)11-4-2-3-5-12(11)22(20)31/h2-5,14-17,28-29,32-33H,6-9,26H2,1H3/t14?,15?,16-,17?,25-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Amrubicin ia an inhibitor of Topoisomerase II Amrubicin. | |||||
| Targets | Topoisomerase II | |||||
| Cell experiment: [1] | |
| Cell lines | CCRF-CEM cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | IC 50: 3.3 µM, 1 hour |
| Applications | Amrubicin induced DNA-protein complex formation in cultured CCRF-CEM cells in a dose-dependent manner. The IC50 value of amrubicin required to inhibit the growth of CCRF-CEM cells was 3.3 µM. Accordingly, under conditions where cell growth was inhibited by amrubicin, considerable amounts of DNA-protein complexes were formed. |
| Animal experiment: [2] | |
| Animal models | BALB/c nu/nu mice bearing Lu-24 or Lu-134 cells |
| Dosage form | Intravenous injection, 25 mg/kg |
| Application | Amrubicin showed significant antitumor activities against both SCLC tumors tested, Lu-24 and Lu-134, with T/C-values at day 14 of 17% and 9%, respectively. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Hanada M, Mizuno S, Fukushima A, et al. A New Antitumor Agent Amrubicin Induces Cell Growth Inhibition by Stabilizing Topoisomerase II-DNA Complex. Cancer Science, 1998, 89(11): 1229-1238. [2] Hanada M, Noguchi T, Yamaoka T. Amrubicin, a novel 9-aminoanthracycline, enhances the antitumor activity of chemotherapeutic agents against human cancer cells in vitro and in vivo. Cancer science, 2007, 98(3): 447-454. | |

Amrubicin Dilution Calculator

Amrubicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0684 mL | 10.3419 mL | 20.6838 mL | 41.3676 mL | 51.7095 mL |
| 5 mM | 0.4137 mL | 2.0684 mL | 4.1368 mL | 8.2735 mL | 10.3419 mL |
| 10 mM | 0.2068 mL | 1.0342 mL | 2.0684 mL | 4.1368 mL | 5.171 mL |
| 50 mM | 0.0414 mL | 0.2068 mL | 0.4137 mL | 0.8274 mL | 1.0342 mL |
| 100 mM | 0.0207 mL | 0.1034 mL | 0.2068 mL | 0.4137 mL | 0.5171 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A synthetic anthracycline antibiotic. It inhibits DNA topoisomerase II. Antineoplastic.
- Ganoderenic acid E
Catalog No.:BCN8241
CAS No.:110241-23-1
- Ganoderic acid N
Catalog No.:BCN2438
CAS No.:110241-19-5
- (-)-beta-Peltatin-5-O-beta-D-glucopyranoside
Catalog No.:BCN3607
CAS No.:11024-59-2
- Digitonin
Catalog No.:BCN3734
CAS No.:11024-24-1
- Nothofagin
Catalog No.:BCN3787
CAS No.:11023-94-2
- Temocapril HCl
Catalog No.:BCC5016
CAS No.:110221-44-8
- Ginsenoside Rc
Catalog No.:BCN1072
CAS No.:11021-14-0
- Ginsenoside Rb2
Catalog No.:BCN1064
CAS No.:11021-13-9
- Cochliophilin A
Catalog No.:BCC8154
CAS No.:110204-45-0
- Malonylginsenoside Rb(1)
Catalog No.:BCC9230
CAS No.:88140-34-5
- 1,5,8-Trihydroxy-3-methoxy-2-prenylxanthone
Catalog No.:BCN1623
CAS No.:110187-11-6
- JZL184
Catalog No.:BCC4790
CAS No.:1101854-58-3
- Agnuside
Catalog No.:BCN5990
CAS No.:11027-63-7
- Bacoside A
Catalog No.:BCC8127
CAS No.:11028-00-5
- CI 966 hydrochloride
Catalog No.:BCC7010
CAS No.:110283-66-4
- Santalol
Catalog No.:BCN8352
CAS No.:11031-45-1
- α-Bungarotoxin
Catalog No.:BCC7264
CAS No.:11032-79-4
- FD-838
Catalog No.:BCN6396
CAS No.:110341-78-1
- CGS 19755
Catalog No.:BCC6986
CAS No.:110347-85-8
- Bavisant dihydrochloride hydrate
Catalog No.:BCC1404
CAS No.:1103522-80-0
- S 32826
Catalog No.:BCC7678
CAS No.:1103672-43-0
- Ch 55
Catalog No.:BCC7241
CAS No.:110368-33-7
- 3-O-Methyltagitinin F
Catalog No.:BCN5991
CAS No.:110382-37-1
- 2,3-Dihydroheveaflavone
Catalog No.:BCN4019
CAS No.:110382-42-8
Lactate dehydrogenase and body mass index are prognostic factors in patients with recurrent small cell lung cancer receiving amrubicin.[Pubmed:26429641]
Tumori. 2016 Dec 1;102(6):606-609.
AIMS AND BACKGROUND: Amrubicin monotherapy can be an effective treatment option for patients with recurrent small cell lung cancer (SCLC). We conducted this retrospective study to investigate the prognostic factors in patients with recurrent SCLC receiving Amrubicin monotherapy. METHODS: The associations between survival and clinical data, including the performance status, body mass index (BMI), plasma lactate dehydrogenase (LDH) level, and plasma neuron-specific enolase level, were evaluated in patients with recurrent SCLC, and a subset analysis of patients with platinum-resistant disease was conducted. RESULTS: In all, 37 patients were evaluated. The median survival from the date of initiation of Amrubicin monotherapy was 9.1 months (95% confidence interval 4.7-12.0 months). Multivariate analysis using a Cox proportional hazard model identified the plasma LDH level (p = 0.049), BMI (p = 0.031), and platinum resistance (p = 0.032) as independent factors associated with survival. The same associations were also observed in the subset of patients with platinum-resistant disease. CONCLUSIONS: Our findings suggest that the plasma LDH level and BMI may be useful prognostic factors in patients with SCLC receiving Amrubicin monotherapy, including patients with platinum-resistant disease.
Comparison of Amrubicin and Weekly Cisplatin/Etoposide/Irinotecan in Patients With Relapsed Small-cell Lung Cancer.[Pubmed:27867001]
Clin Lung Cancer. 2017 Mar;18(2):234-240.e2.
BACKGROUND: Although several agents have been introduced for the treatment of relapsed small-cell lung cancer (SCLC), there is still only limited evidence regarding second- and later-line chemotherapies for these patients. PATIENTS AND METHODS: Consecutive patients with relapsed SCLC treated at the National Cancer Center Hospital between 2000 and 2014 were analyzed. Patients' characteristics and treatments to explore factors associated with the survival outcomes were reviewed. RESULTS: A total of 580 patients diagnosed as having SCLC received first-line chemotherapy/chemoradiotherapy, of which 343 (59%) received second-line chemotherapy. Among the 343 patients, 193, 148, and 2 patients were diagnosed sensitive relapse, refractory relapse, and relapse of unknown sensitivity status, respectively. Second-line chemotherapy regimens used were as follows: Amrubicin (AMR) in 188 (55%) patients; weekly cisplatin/etoposide/irinotecan (PEI) in 56 (16%) patients; topotecan in 18 (5.2%) patients; others in 81 (24%) patients. In the analysis including all patients, the following outcomes were obtained for the patients treated with AMR and PEI, respectively: objective response rate: 51% and 73%; median progression-free survival: 4.5 and 4.2 months; median overall survival: 10.0 and 10.8 months. Multivariate analysis identified sensitive relapse to first-line treatment (vs. refractory relapse) (P = .007) and AMR as second-line treatment (vs. PEI) (P = .005) as independent favorable prognostic factors for survival. CONCLUSION: AMR showed a favorable trend compared with PEI in terms of the progression-free survival and feasibility in SCLC patients with relapsed disease. Based on our findings, we suggest that a randomized trial comparing AMR and PEI is warranted.
Potential Activity of Amrubicin as a Salvage Therapy for Merkel Cell Carcinoma.[Pubmed:28250307]
Intern Med. 2017;56(5):567-570.
Merkel cell carcinoma (MCC) is a rare neuroendocrine carcinoma of the skin with an aggressive clinical course. Although anthracycline- and platinum-based regimens are empirically used as first-line treatments for metastatic or unresectable cases, no salvage therapy has been established. A 73-year-old man with platinum-refractory recurrent MCC was treated with Amrubicin. The symptoms improved soon, and a partial response was achieved. A total of nine cycles of Amrubicin were administered in nine months with manageable adverse events until disease progression was finally observed. The present findings suggest the potential of Amrubicin monotherapy as a second-line therapy for patients with advanced/recurrent MCC.
METASTATIC SMALL CELL CARCINOMA OF THE URINARY BLADDER TREATED WITH SYSTEMIC CHEMOTHERAPY INCLUDING AN AMRUBICIN; A CASE REPORT.[Pubmed:28132989]
Nihon Hinyokika Gakkai Zasshi. 2016;107(1):34-38.
We report a 59-year-old male patient with metastatic small cell carcinoma of the bladder treated with systemic chemotherapy including an Amrubicin. The patient was referred to our hospital complaining of macrohematuria. A cytoscopy revealed a non-papillary, broad-based tumor extending from the right to the posterior wall of the bladder. A computed tomography showed bilateral hydronephrosis caused by the bladder tumor and multiple metastases to the para-aortic and common iliac lymph nodes. The histopathological findings following a transurethral resection of the bladder tumor revealed a T2N3M1, LYM, stage IV small cell carcinoma. We administered two courses of systemic chemotherapy consisting of cisplatin (CDDP) plus an etoposide (VP-16), a first-line treatment usually administered to patients with small cell carcinoma of the lung. We then administered second-line chemotherapy consisting of CDDP plus an irinotecan. When the first and second-line therapies failed to halt progression of the disease, we decided to use Amrubicin as the third-line therapy concomitant with radiotherapy for local control. Although the NSE (neuron-specific enolase) value decreased, the patient died 11 months after the initial examination. To our knowledge, this is the first case in which small cell carcinoma of the bladder was treated with Amrubicin.


