DigitoninCAS# 11024-24-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 11024-24-1 | SDF | Download SDF |
| PubChem ID | 439305 | Appearance | Powder |
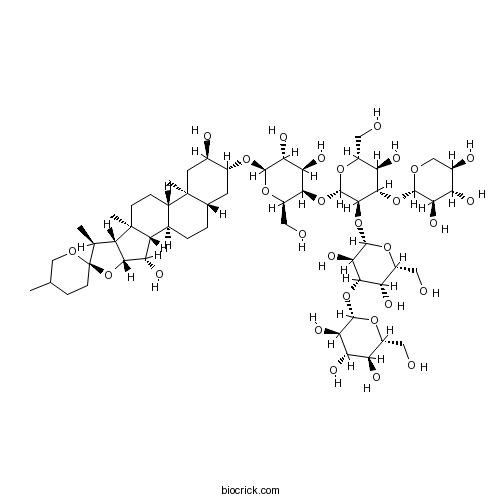
| Formula | C56H92O29 | M.Wt | 1229.3 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | H2O : 50 mg/mL (40.67 mM; Need ultrasonic) | ||
| SMILES | CC1CCC2(C(C3C(O2)C(C4C3(CCC5C4CCC6C5(CC(C(C6)OC7C(C(C(C(O7)CO)OC8C(C(C(C(O8)CO)O)OC9C(C(C(CO9)O)O)O)OC2C(C(C(C(O2)CO)O)OC2C(C(C(C(O2)CO)O)O)O)O)O)O)O)C)C)O)C)OC1 | ||
| Standard InChIKey | UVYVLBIGDKGWPX-QYLWGQLPSA-N | ||
| Standard InChI | InChI=1S/C56H92O29/c1-19-7-10-56(75-17-19)20(2)31-45(85-56)37(67)32-22-6-5-21-11-26(24(61)12-55(21,4)23(22)8-9-54(31,32)3)76-50-42(72)39(69)44(30(16-60)80-50)81-53-48(47(36(66)29(15-59)79-53)83-49-40(70)33(63)25(62)18-74-49)84-52-43(73)46(35(65)28(14-58)78-52)82-51-41(71)38(68)34(64)27(13-57)77-51/h19-53,57-73H,5-18H2,1-4H3/t19?,20-,21-,22+,23-,24+,25+,26+,27+,28+,29+,30+,31-,32+,33-,34+,35-,36+,37-,38-,39+,40+,41+,42+,43+,44-,45+,46-,47-,48+,49-,50+,51-,52-,53-,54+,55-,56+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Digitonin alone and together with the set of secondary metabolites (SM) , can mediate MDR reversal in cancer cells. 2. Digitonin solubilizes mitochondrial membrane, breaks the integrity of the respiratory chain and releases two mobile redox-active components: coenzyme Q (CoQ) and cytochrome c (cyt c). |
| Targets | P-gp |

Digitonin Dilution Calculator

Digitonin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.8135 mL | 4.0674 mL | 8.1347 mL | 16.2694 mL | 20.3368 mL |
| 5 mM | 0.1627 mL | 0.8135 mL | 1.6269 mL | 3.2539 mL | 4.0674 mL |
| 10 mM | 0.0813 mL | 0.4067 mL | 0.8135 mL | 1.6269 mL | 2.0337 mL |
| 50 mM | 0.0163 mL | 0.0813 mL | 0.1627 mL | 0.3254 mL | 0.4067 mL |
| 100 mM | 0.0081 mL | 0.0407 mL | 0.0813 mL | 0.1627 mL | 0.2034 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nothofagin
Catalog No.:BCN3787
CAS No.:11023-94-2
- Temocapril HCl
Catalog No.:BCC5016
CAS No.:110221-44-8
- Ginsenoside Rc
Catalog No.:BCN1072
CAS No.:11021-14-0
- Ginsenoside Rb2
Catalog No.:BCN1064
CAS No.:11021-13-9
- Cochliophilin A
Catalog No.:BCC8154
CAS No.:110204-45-0
- Malonylginsenoside Rb(1)
Catalog No.:BCC9230
CAS No.:88140-34-5
- 1,5,8-Trihydroxy-3-methoxy-2-prenylxanthone
Catalog No.:BCN1623
CAS No.:110187-11-6
- JZL184
Catalog No.:BCC4790
CAS No.:1101854-58-3
- Ouabain Octahydrate
Catalog No.:BCC5211
CAS No.:11018-89-6
- 4-Galloylquinic acid
Catalog No.:BCN3733
CAS No.:110170-37-1
- Methyl hesperidin
Catalog No.:BCN6341
CAS No.:11013-97-1
- Indoximod (NLG-8189)
Catalog No.:BCC5584
CAS No.:110117-83-4
- (-)-beta-Peltatin-5-O-beta-D-glucopyranoside
Catalog No.:BCN3607
CAS No.:11024-59-2
- Ganoderic acid N
Catalog No.:BCN2438
CAS No.:110241-19-5
- Ganoderenic acid E
Catalog No.:BCN8241
CAS No.:110241-23-1
- Amrubicin
Catalog No.:BCC3640
CAS No.:110267-81-7
- Agnuside
Catalog No.:BCN5990
CAS No.:11027-63-7
- Bacoside A
Catalog No.:BCC8127
CAS No.:11028-00-5
- CI 966 hydrochloride
Catalog No.:BCC7010
CAS No.:110283-66-4
- Santalol
Catalog No.:BCN8352
CAS No.:11031-45-1
- α-Bungarotoxin
Catalog No.:BCC7264
CAS No.:11032-79-4
- FD-838
Catalog No.:BCN6396
CAS No.:110341-78-1
- CGS 19755
Catalog No.:BCC6986
CAS No.:110347-85-8
- Bavisant dihydrochloride hydrate
Catalog No.:BCC1404
CAS No.:1103522-80-0
Influence of combinations of digitonin with selected phenolics, terpenoids, and alkaloids on the expression and activity of P-glycoprotein in leukaemia and colon cancer cells.[Pubmed:23999162]
Phytomedicine. 2013 Dec 15;21(1):47-61.
P-glycoprotein (P-gp or MDR1) is an ATP-binding cassette (ABC) transporter. It is involved in the efflux of several anticancer drugs, which leads to chemotherapy failure and multidrug resistance (MDR) in cancer cells. Representative secondary metabolites (SM) including phenolics (EGCG and thymol), terpenoids (menthol, aromadendrene, beta-sitosterol-O-glucoside, and beta-carotene), and alkaloids (glaucine, harmine, and sanguinarine) were evaluated as potential P-gp inhibitors (transporter activity and expression level) in P-gp expressing Caco-2 and CEM/ADR5000 cancer cell lines. Selected SM increased the accumulation of the rhodamine 123 (Rho123) and calcein-AM (CAM) in a dose dependent manner in Caco-2 cells, indicating that they act as competitive inhibitors of P-gp. Non-toxic concentrations of beta-carotene (40muM) and sanguinarine (1muM) significantly inhibited Rho123 and CAM efflux in CEM/ADR5000 cells by 222.42% and 259.25% and by 244.02% and 290.16%, respectively relative to verapamil (100%). Combination of the saponin Digitonin (5muM), which also inhibits P-gp, with SM significantly enhanced the inhibition of P-gp activity. The results were correlated with the data obtained from a quantitative analysis of MDR1 expression. Both compounds significantly decreased mRNA levels of the MDR1 gene to 48% (p<0.01) and 46% (p<0.01) in Caco-2, and to 61% (p<0.05) and 1% (p<0.001) in CEM/ADR5000 cells, respectively as compared to the untreated control (100%). Combinations of Digitonin with SM resulted in a significant down-regulation of MDR1. Our findings provide evidence that the selected SM interfere directly and/or indirectly with P-gp function. Combinations of different P-gp substrates, such as Digitonin alone and together with the set of SM, can mediate MDR reversal in cancer cells.
Synergism of three-drug combinations of sanguinarine and other plant secondary metabolites with digitonin and doxorubicin in multi-drug resistant cancer cells.[Pubmed:23146422]
Phytomedicine. 2012 Nov 15;19(14):1288-97.
We determined the ability of some phytochemicals, including alkaloids (glaucine, harmine, and sanguinarine), phenolics (EGCG and thymol), and terpenoids (menthol, aromadendrene, beta-sitosterol-O-glucoside, and beta-carotene), alone or in combination with the saponin Digitonin to reverse the relative multi-drug resistance of Caco-2 and CEM/ADR5000 cells to the chemotherapeutical agent doxorubicin. The IC(50) of doxorubicin in Caco-2 and CEM/ADR5000 was 4.22 and 44.08muM, respectively. Combination of non-toxic concentrations of individual secondary metabolite with doxorubicin synergistically sensitized Caco-2 and CEM/ADR5000 cells, and significantly enhanced the cytotoxicity of doxorubicin. Furthermore, three-drug combinations (secondary metabolite+Digitonin+doxorubicin) were even more powerful. The best synergist was the benzophenanthridine alkaloid sanguinarine. It reduced the IC(50) value of doxorubicin 17.58-fold in two-drug combinations (sanguinarine+doxorubicin) and even 35.17-fold in three-drug combinations (sanguinarine+Digitonin+doxorubicin) in Caco-2 cells. Thus synergistic drug combinations offer the possibility to enhance doxorubicin efficacy in chemotherapy.
Analysis of nucleocytoplasmic transport in digitonin-permeabilized cells under different cellular conditions.[Pubmed:24857737]
Methods Cell Biol. 2014;122:331-52.
The regulation of nucleocytoplasmic transport is crucial not only for basic cellular activities but also for physiological adaptation to specific situation during the cell cycle, development, or stress. Although a wide variety of transport pathways have been identified in eukaryotic cells, the functional significance of their multiplicity remains unclear. The best-characterized nuclear transport receptors (NTRs) are the members of the importin beta family (karyopherin, transportin) whose association with specific cargoes is regulated by the GTPase Ran. In this chapter, we first provide an overview of the various expression vectors used to purify recombinant NTRs. We then describe two sets of recent examples of using well-established Digitonin-permeabilized cell-free transport systems in mammalian cells to mimic different cellular conditions in living cells: normal/heat-shock conditions and interphase/mitosis. In the former case, physiological regulation impacts different transport pathways in opposite ways. In the latter case, the importin beta-Ran system is used at different cell-cycle stages but with the same biochemical principle to specify the nuclear localization and chromatin loading of a specific protein, respectively. This in vitro transport assay, when adapted to specific cellular conditions or particular substrates, should help to uncover specific transport pathways or transport factors function under different cellular conditions.
Digitonin synergistically enhances the cytotoxicity of plant secondary metabolites in cancer cells.[Pubmed:23062361]
Phytomedicine. 2012 Nov 15;19(14):1307-14.
In phytotherapy, extracts from medicinal plants are employed which contain mixtures of secondary metabolites. Their modes of action are complex because the secondary metabolites can react with single or multiple targets. The components in a mixture can exert additive or even synergistic activities. In this study, the cytotoxicity of some phytochemicals, including phenolics (EGCG and thymol), terpenoids (menthol, aromadendrene, beta-sitosterol-O-glucoside, and beta-carotene) and alkaloids (glaucine, harmine, and sanguinarine) were investigated alone or in combination with the cytotoxic monodesmosidic steroidal saponin Digitonin in Caco-2, MCF-7, CEM/ADR5000, and CCRF-CEM cells. Digitonin was combined in non-toxic concentrations (5muM in each cell line; except in MCF-7 the concentration was 2muM), together with a selection of phenolics, terpenoids, and alkaloids to evaluate potential synergistic or additive effects. An enhanced cytotoxicity was observed in most combinations. Even multi-drug resistant (MDR) cells (such as CEM/ADR5000 cells), with a high expression of P-glycoprotein, were responsive to combinations. Sanguinarine was the most cytotoxic alkaloid against CEM/ADR5000, MCF-7, and CCRF-CEM cells alone and in combination with Digitonin. As compared to sanguinarine alone, the combination was 44.53-, 15.38-, and 6.65-fold more toxic in each cell line, respectively. Most combinations synergistically increased the cytotoxicity, stressing the importance of synergy when using multi-target drugs and mixtures in phytotherapy.
Idebenone-induced recovery of glycerol-3-phosphate and succinate oxidation inhibited by digitonin.[Pubmed:22480420]
Physiol Res. 2012;61(3):259-65. Epub 2012 Apr 5.
Digitonin solubilizes mitochondrial membrane, breaks the integrity of the respiratory chain and releases two mobile redox-active components: coenzyme Q (CoQ) and cytochrome c (cyt c). In the present study we report the inhibition of glycerol-3-phosphate- and succinate-dependent oxygen consumption rates by Digitonin treatment. Our results show that the inhibition of oxygen consumption rates is recovered by the addition of exogenous synthetic analog of CoQ idebenone (hydroxydecyl-ubiquinone; IDB) and cyt c. Glycerol-3-phosphate oxidation rate is recovered to 148 % of control values, whereas succinate-dependent oxidation rate only to 68 %. We find a similar effect on the activities of glycerol-3-phosphate and succinate cytochrome c oxidoreductase. Our results also indicate that succinate-dependent oxidation is less sensitive to Digitonin treatment and less activated by IDB in comparison with glycerol-3-phosphate-dependent oxidation. These findings might indicate the different mechanism of the electron transfer from two flavoprotein-dependent dehydrogenases (glycerol-3-phosphate dehydrogenase and succinate dehydrogenase) localized on the outer and inner face of the inner mitochondrial membrane, respectively.


