Calystegine A3CAS# 131580-36-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 131580-36-4 | SDF | Download SDF |
| PubChem ID | 442999 | Appearance | Powder |
| Formula | C7H13NO3 | M.Wt | 159.18 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
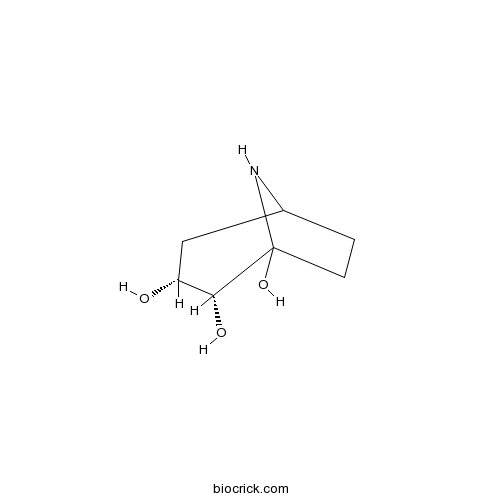
| Chemical Name | (3R,4R)-8-azabicyclo[3.2.1]octane-3,4,5-triol | ||
| SMILES | C1CC2(C(C(CC1N2)O)O)O | ||
| Standard InChIKey | XOCBOVUINUHZJA-RKXXOXFUSA-N | ||
| Standard InChI | InChI=1S/C7H13NO3/c9-5-3-4-1-2-7(11,8-4)6(5)10/h4-6,8-11H,1-3H2/t4?,5-,6-,7?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Calystegines A3 and B2 can effectively stabilize β-glucocerebrosidase, and consequently increase intracellular β-glucocerebrosidase activities in N370S fibroblasts. 2. Calystegines A3, B2, and C1 are glycosidase inhibitors. |

Calystegine A3 Dilution Calculator

Calystegine A3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.2822 mL | 31.411 mL | 62.822 mL | 125.6439 mL | 157.0549 mL |
| 5 mM | 1.2564 mL | 6.2822 mL | 12.5644 mL | 25.1288 mL | 31.411 mL |
| 10 mM | 0.6282 mL | 3.1411 mL | 6.2822 mL | 12.5644 mL | 15.7055 mL |
| 50 mM | 0.1256 mL | 0.6282 mL | 1.2564 mL | 2.5129 mL | 3.1411 mL |
| 100 mM | 0.0628 mL | 0.3141 mL | 0.6282 mL | 1.2564 mL | 1.5705 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Camelliaside B
Catalog No.:BCN3872
CAS No.:131573-90-5
- Triumbelletin
Catalog No.:BCN6779
CAS No.:131559-54-1
- VU 591 hydrochloride
Catalog No.:BCC6126
CAS No.:1315380-70-1
- NPEC-caged-(1S,3R)-ACPD
Catalog No.:BCC7653
CAS No.:1315379-60-2
- Rac1 Inhibitor F56, control peptide
Catalog No.:BCC5887
CAS No.:1315378-77-8
- pep2-SVKE
Catalog No.:BCC5785
CAS No.:1315378-76-7
- MNI caged kainic acid
Catalog No.:BCC7297
CAS No.:1315378-75-6
- Bax inhibitor peptide, negative control
Catalog No.:BCC2395
CAS No.:1315378-74-5
- PDZ1 Domain inhibitor peptide
Catalog No.:BCC5883
CAS No.:1315378-73-4
- Scrambled 10Panx
Catalog No.:BCC1246
CAS No.:1315378-72-3
- TCS 184
Catalog No.:BCC5899
CAS No.:1315378-71-2
- pep2-AVKI
Catalog No.:BCC5787
CAS No.:1315378-69-8
- Betulinaldehyde
Catalog No.:BCN1249
CAS No.:13159-28-9
- Amyloid Beta-peptide (25-35) (human)
Catalog No.:BCC1027
CAS No.:131602-53-4
- (+)-Pteryxin
Catalog No.:BCN3470
CAS No.:13161-75-6
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- ACY-241
Catalog No.:BCC6460
CAS No.:1316215-12-9
- OPC 21268
Catalog No.:BCC7812
CAS No.:131631-89-5
- Chalepensin
Catalog No.:BCN7334
CAS No.:13164-03-9
- Turmeronol A
Catalog No.:BCN6777
CAS No.:131651-37-1
- 8-(1-Chloro-2-hydroxy-3-methylbut-3-enyl)-7-methoxycoumarin
Catalog No.:BCN7058
CAS No.:131652-35-2
- Parsonsianine
Catalog No.:BCN2110
CAS No.:131683-36-8
- (R)-(-)-4-Benzyl-3-propionyl-2-oxazolidinone
Catalog No.:BCC8392
CAS No.:131685-53-5
- Arbidol HCl
Catalog No.:BCC3722
CAS No.:131707-23-8
Docking and SAR studies of calystegines: binding orientation and influence on pharmacological chaperone effects for Gaucher's disease.[Pubmed:24657053]
Bioorg Med Chem. 2014 Apr 15;22(8):2435-41.
We report on the identification of the required configuration and binding orientation of nor-tropane alkaloid calystegines against beta-glucocerebrosidase. Calystegine B2 is a potent competitive inhibitor of human lysosomal beta-glucocerebrosidase with Ki value of 3.3 muM. A molecular docking study revealed that calystegine B2 had a favorable van der Waals interactions (Phe128, Trp179, and Phe246) and the hydrogen bonding (Glu235, Glu340, Asp127, Trp179, Asn234, Trp381 and Asn396) was similar to that of isofagomine. All calystegine isomers bound into the same active site as calystegine B2 and the essential hydrogen bonds formed to Asp127, Glu235 and Glu340 were maintained. However, their binding orientations were obviously different. Calystegine A3 bound to beta-glucocerebrosidase with the same orientations as calystegine B2 (Type 1), while calystegine B3 and B4 had different binding orientations (Type 2). It is noteworthy that Type 1 orientated calystegines B2 and A3 effectively stabilized beta-glucocerebrosidase, and consequently increased intracellular beta-glucocerebrosidase activities in N370S fibroblasts, while Type 2 orientated calystegines B3 and B4 could not keep the enzyme activity. These results clearly indicate that the binding orientations of calystegines are changed by the configuration of the hydroxyl groups on the nor-tropane ring and the suitable binding orientation is a requirement for achieving a strong affinity to beta-glucocerebrosidase.
The comparative pathology of the glycosidase inhibitors swainsonine, castanospermine, and calystegines A3, B2, and C1 in mice.[Pubmed:18497426]
Toxicol Pathol. 2008 Jul;36(5):651-9.
To study various polyhydroxy-alkaloid glycosidase inhibitors, 16 groups of 3 mice were dosed using osmotic minipumps with swainsonine (0, 0.1, 1, and 10 mg/kg/day), castanospermine, and calystegines A(3), B(2), and C(1) (1, 10, and 100 mg/kg/day). After 28 days, the mice were euthanized, necropsied, and examined using light and electron microscopy. The high-dose swainsonine-treated mice developed neurologic disease with neuro-visceral vacuolation typical of locoweed poisoning. Castanospermine- and calystegines-treated mice were clinically normal; however, high-dose castanospermine-treated mice had thyroid, renal, hepatic, and skeletal myocyte vacuolation. Histochemically, swainsonine- and castanospermine-induced vacuoles contained mannose-rich oligosaccharides. High-dose calystegine A(3)-treated mice had increased numbers of granulated cells in the hepatic sinusoids. Electron microscopy, lectin histochemistry, and immunohistochemistry suggest these are pit cells (specialized NK cells). Histochemically, the granules contain glycoproteins or oligosaccharides with abundant terminal N-acetylglucosamine residues. Other calystegine-treated mice were histologically normal. These findings indicate that swainsonine produced lesions similar to locoweed, castanospermine caused vacuolar changes with minor changes in glycogen metabolism, and only calystegine A(3) produced minimal hepatic changes. These also suggest that in mice calystegines and castanospermine are less toxic than swainsonine, and as rodents are relatively resistant to disease, they are poor models to study such induced storage diseases.
Chiral pool synthesis of calystegine A3 from 2-deoxyglucose via a Brown allylation.[Pubmed:22088883]
Carbohydr Res. 2011 Dec 27;346(18):2855-61.
Calystegine A(3) is a naturally occurring nortropane iminosugar of which there previously have been three total syntheses. Inspired by our previous work we here report on a fourth approach using 17 steps from 2-deoxy-d-glucose applying a diastereoselective allylation protocol.


