CercosporamideMnk2 and JAK3 inhibitor CAS# 131436-22-1 |
- Ruxolitinib sulfate
Catalog No.:BCC1913
CAS No.:1092939-16-6
- GLPG0634
Catalog No.:BCC4145
CAS No.:1206161-97-8
- TCS 21311
Catalog No.:BCC2443
CAS No.:1260181-14-3
- WHI-P154
Catalog No.:BCC2202
CAS No.:211555-04-3
- ZM 449829
Catalog No.:BCC2444
CAS No.:4452-06-6
- Tofacitinib (CP-690550) Citrate
Catalog No.:BCC2189
CAS No.:540737-29-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 131436-22-1 | SDF | Download SDF |
| PubChem ID | 131379 | Appearance | Powder |
| Formula | C16H13NO7 | M.Wt | 331.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (-)-Cercosporamide | ||
| Solubility | Soluble to 10 mM in DMSO | ||
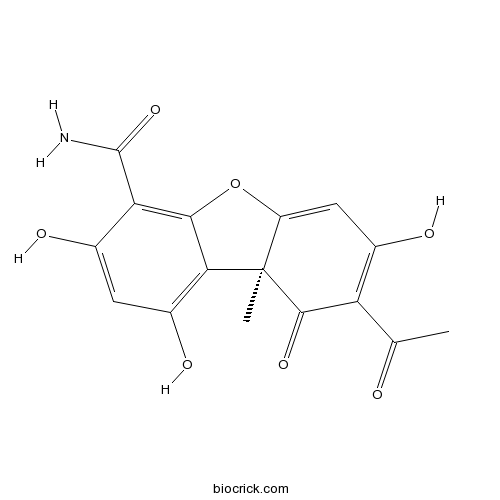
| Chemical Name | (9aS)-8-acetyl-1,3,7-trihydroxy-9a-methyl-9-oxodibenzofuran-4-carboxamide | ||
| SMILES | CC(=O)C1=C(C=C2C(C1=O)(C3=C(C=C(C(=C3O2)C(=O)N)O)O)C)O | ||
| Standard InChIKey | GEWLYFZWVLXQME-MRXNPFEDSA-N | ||
| Standard InChI | InChI=1S/C16H13NO7/c1-5(18)10-7(20)4-9-16(2,14(10)22)12-8(21)3-6(19)11(15(17)23)13(12)24-9/h3-4,19-21H,1-2H3,(H2,17,23)/t16-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of MAP-kinase interacting kinase-2 (Mnk2) and JAK3 (IC50 values are 11, 31 and 116 nM for Mnk2, JAK3 and Mnk1 respectively). Blocks eIF4E phosphorylation; exhibits antiproliferative and proapoptotic activity in cancer cells in vitro. Also inhibits Pkc1. Orally bioavailable. |

Cercosporamide Dilution Calculator

Cercosporamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0186 mL | 15.093 mL | 30.1859 mL | 60.3719 mL | 75.4649 mL |
| 5 mM | 0.6037 mL | 3.0186 mL | 6.0372 mL | 12.0744 mL | 15.093 mL |
| 10 mM | 0.3019 mL | 1.5093 mL | 3.0186 mL | 6.0372 mL | 7.5465 mL |
| 50 mM | 0.0604 mL | 0.3019 mL | 0.6037 mL | 1.2074 mL | 1.5093 mL |
| 100 mM | 0.0302 mL | 0.1509 mL | 0.3019 mL | 0.6037 mL | 0.7546 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Potent inhibitor of MAP-kinase interacting kinase-2 (Mnk2) and JAK3 (IC50 values are 11, 31 and 116 nM for Mnk2, JAK3 and Mnk1 respectively). Blocks eIF4E phosphorylation; exhibits antiproliferative and proapoptotic activity in cancer cells in vitro. Also inhibits Pkc1. Orally bioavailable.
- UNC669
Catalog No.:BCC3997
CAS No.:1314241-44-5
- TC-N 22A
Catalog No.:BCC6150
CAS No.:1314140-00-5
- Hemopressin (human, mouse)
Catalog No.:BCC6065
CAS No.:1314035-51-2
- CBZ-Osu
Catalog No.:BCC2798
CAS No.:13139-17-8
- Boc-Ile-OH.1/2H2O
Catalog No.:BCC3406
CAS No.:13139-16-7
- Boc-Leu-OH.H2O
Catalog No.:BCC3408
CAS No.:13139-15-6
- Boc-Trp-OH
Catalog No.:BCC3455
CAS No.:13139-14-5
- Teijin compound 1
Catalog No.:BCC6057
CAS No.:1313730-14-1
- Periplocin
Catalog No.:BCN2655
CAS No.:13137-64-9
- erythro-Guaiacylglycerol beta-threo-syringylglycerol ether
Catalog No.:BCN7333
CAS No.:1313434-74-0
- NVP-CGM097
Catalog No.:BCC5395
CAS No.:1313363-54-0
- Nystose
Catalog No.:BCN5397
CAS No.:13133-07-8
- Amyloid Beta-Peptide (1-40) (human)
Catalog No.:BCC1045
CAS No.:131438-79-4
- TMP269
Catalog No.:BCC3993
CAS No.:1314890-29-3
- HG6-64-1
Catalog No.:BCC5459
CAS No.:1315329-43-1
- B-Raf inhibitor
Catalog No.:BCC1437
CAS No.:1315330-11-0
- NG25
Catalog No.:BCC1799
CAS No.:1315355-93-1
- pep2-EVKI
Catalog No.:BCC5786
CAS No.:1315378-67-6
- pep2-AVKI
Catalog No.:BCC5787
CAS No.:1315378-69-8
- TCS 184
Catalog No.:BCC5899
CAS No.:1315378-71-2
- Scrambled 10Panx
Catalog No.:BCC1246
CAS No.:1315378-72-3
- PDZ1 Domain inhibitor peptide
Catalog No.:BCC5883
CAS No.:1315378-73-4
- Bax inhibitor peptide, negative control
Catalog No.:BCC2395
CAS No.:1315378-74-5
- MNI caged kainic acid
Catalog No.:BCC7297
CAS No.:1315378-75-6
Synthesis and biological evaluation of novel (-)-Cercosporamide derivatives as potent selective PPARgamma modulators.[Pubmed:22727448]
Eur J Med Chem. 2012 Aug;54:522-33.
Selective peroxisome proliferator-activated receptor gamma (PPARgamma) modulators are expected to be a novel class of drugs improving plasma glucose levels without PPARgamma-related adverse effects. As a continuation of our studies for (-)-Cercosporamide derivatives as selective PPARgamma modulators, we synthesized substituted naphthalene type compounds and identified the most potent compound 15 (EC(50) = 0.94 nM, E(max) = 38%). Compound 15 selectively activated PPARgamma transcription and did not activate PPARalpha and PPARdelta. The potassium salt of compound 15 showed a high solubility and a good oral bioavailability (58%). Oral administration of the potassium salt remarkably improved the plasma glucose levels of female Zucker diabetic fatty rats at 1 mg/kg. Moreover, it did not cause a plasma volume increase or a cardiac enlargement in Wistar-Imamichi rats, even at 100 mg/kg.
Synthesis and antiproliferative activity of benzofuran-based analogs of cercosporamide against non-small cell lung cancer cell lines.[Pubmed:24121233]
Eur J Med Chem. 2013 Nov;69:823-32.
A novel series of 3-methyl-1-benzofuran derivatives were synthesized and screened in vitro for their antiproliferative activity against two human NSCLC cell lines (NSCLC-N6 mutant p53 and A549 wild type p53). Most promising compounds presented a structural analogy with the west part of Cercosporamide, a natural product of biological interest. In particular, compounds 10, 12 and 31 showed cytotoxic activities at micromolar concentrations (IC(5)(0) < 9.3 muM) and compounds 13, 18 and 32 displayed moderate IC(5)(0) values (25-40 muM).
Inhibition of eukaryotic initiation factor 4E phosphorylation by cercosporamide selectively suppresses angiogenesis, growth and survival of human hepatocellular carcinoma.[Pubmed:27662474]
Biomed Pharmacother. 2016 Dec;84:237-243.
Mnk kinase is required for the phosphorylation and activation of the eukaryotic initiation factor 4E (eIF4E), which regulates translation of proteins involve in important aspects of hepatocellular carcinoma (HCC). Here we investigated whether an antifungal agent, Cercosporamide, which had been recently identified as a potent Mnk inhibitor, is active against HCC and angiogenesis. We showed that Cercosporamide significantly inhibited growth and induced caspase-dependent apoptosis on numerous HCC cell lines, while sparing normal liver cells. In addition, Cercosporamide impaired HCC angiogenesis via inhibiting HCC-endothelial cells (HCC-EC) capillary network formation, migration, proliferation and survival. Importantly, Cercosporamide sensitized HCC cells to cisplatin in in vitro cell culture and in vivo HCC xenograft mouse model. Cercosporamide blocked the phosphorylation of eIF4E but not Erk or p38 in a dose- and time-dependent manner in HCC and HCC-EC cells, suggesting that suppression of eIF4E phosphorylation was the result of inhibition of Mnk but not Mnk upstream pathways. Overexpression of constitutively active eIF4E (S209D) but not the nonphosphorylatable eIF4E (S209A) abolished the inhibitory effects of Cercosporamide in HepG2 cells. Altogether, our work demonstrates that Cercosporamide acts as a Mnk inhibitor through blockage of eIF4E phosphorylation and selectively exhibits anti-HCC activities. Our work suggests that targeting MNK-eIF4E pathway represents a therapeutic strategy to overcome chemo-resistance for HCC treatment.
Inhibition of Mnk kinase activity by cercosporamide and suppressive effects on acute myeloid leukemia precursors.[Pubmed:23509154]
Blood. 2013 May 2;121(18):3675-81.
Mnk kinases regulate the phosphorylation and activation of the eukaryotic initiation factor 4E (eIF4E), a protein that plays key roles in the initiation of messenger RNA translation and whose activity is critical for various cellular functions. eIF4E is deregulated in acute myeloid leukemia (AML), and its aberrant activity contributes to leukemogenesis. We determined whether Cercosporamide, an antifungal agent that was recently shown to act as a unique Mnk inhibitor, exhibits antileukemic properties. Treatment of AML cells with Cercosporamide resulted in a dose-dependent suppression of eIF4E phosphorylation. Such suppression of Mnk kinase activity and eIF4E phosphorylation by Cercosporamide resulted in dose-dependent suppressive effects on primitive leukemic progenitors (CFU-L) from AML patients and enhanced the antileukemic properties of cytarabine (Ara-C) or mammalian target of rapamycin (mTOR) complex 1 inhibition. Similarly, the combination of Cercosporamide with cytarabine resulted in enhanced antileukemic responses in a xenograft mouse model in vivo. Altogether, this work demonstrates that the unique Mnk inhibitor Cercosporamide suppresses phosphorylation of eIF4E and exhibits antileukemic effects, in support of future clinical-translational efforts involving combinations of Mnk inhibitors with cytarabine and/or mTOR inhibitors for the treatment of AML.
Targeting Mnks for cancer therapy.[Pubmed:22392765]
Oncotarget. 2012 Feb;3(2):118-31.
Deregulation of protein synthesis is a common event in human cancer and a key player in translational control is eIF4E. Elevated expression levels of eIF4E promote cancer development and progression. Recent findings suggest that eIF4E activity is a key determinant of the PI3K/Akt/mTOR and Ras/Raf/MEK/ERK mediated tumorigenic activity and targeting eIF4E should have a major impact on these pathways in human cancer. The function of eIF4E is modulated through phosphorylation of a conserved serine (Ser209) by Mnk1 and Mnk2 downstream of ERK. While the phosphorylation event is necessary for oncogenic transformation, it seems to be dispensable for normal development. Hence, pharmacologic Mnk inhibitors may provide non-toxic and effective anti-cancer strategy. Strong circumstantial evidence indicates that Mnk inhibition presents attractive therapeutic potential, but the lack of selective Mnk inhibitors has so far confounded pharmacological target validation and clinical development.
Therapeutic inhibition of MAP kinase interacting kinase blocks eukaryotic initiation factor 4E phosphorylation and suppresses outgrowth of experimental lung metastases.[Pubmed:21233335]
Cancer Res. 2011 Mar 1;71(5):1849-57.
Activation of the translation initiation factor 4E (eIF4E) promotes malignant transformation and metastasis. Signaling through the AKT-mTOR pathway activates eIF4E by phosphorylating the inhibitory 4E binding proteins (4E-BP). This liberates eIF4E and allows binding to eIF4G. eIF4E can then be phosphorylated at serine 209 by the MAPK-interacting kinases (Mnk), which also interact with eIF4G. Although dispensable for normal development, Mnk function and eIF4E phosphorylation promote cellular proliferation and survival and are critical for malignant transformation. Accordingly, Mnk inhibition may serve as an attractive cancer therapy. We now report the identification of a potent, selective and orally bioavailable Mnk inhibitor that effectively blocks 4E phosphorylation both in vitro and in vivo. In cultured cancer cell lines, Mnk inhibitor treatment induces apoptosis and suppresses proliferation and soft agar colonization. Importantly, a single, orally administered dose of this Mnk inhibitor substantially suppresses eIF4E phosphorylation for at least 4 hours in human xenograft tumor tissue and mouse liver tissue. Moreover, oral dosing with the Mnk inhibitor significantly suppresses outgrowth of experimental B16 melanoma pulmonary metastases as well as growth of subcutaneous HCT116 colon carcinoma xenograft tumors, without affecting body weight. These findings offer the first description of a novel, orally bioavailable MNK inhibitor and the first preclinical proof-of-concept that MNK inhibition may provide a tractable cancer therapeutic approach.
Discovery of cercosporamide, a known antifungal natural product, as a selective Pkc1 kinase inhibitor through high-throughput screening.[Pubmed:15302826]
Eukaryot Cell. 2004 Aug;3(4):932-43.
The Pkc1-mediated cell wall integrity-signaling pathway is highly conserved in fungi and is essential for fungal growth. We thus explored the potential of targeting the Pkc1 protein kinase for developing broad-spectrum fungicidal antifungal drugs through a Candida albicans Pkc1-based high-throughput screening. We discovered that Cercosporamide, a broad-spectrum natural antifungal compound, but previously with an unknown mode of action, is actually a selective and highly potent fungal Pkc1 kinase inhibitor. This finding provides a molecular explanation for previous observations in which Saccharomyces cerevisiae cell wall mutants were found to be highly sensitive to Cercosporamide. Indeed, S. cerevisiae mutant cells with reduced Pkc1 kinase activity become hypersensitive to Cercosporamide, and this sensitivity can be suppressed under high-osmotic growth conditions. Together, the results demonstrate that Cercosporamide acts selectively on Pkc1 kinase and, thus, they provide a molecular mechanism for its antifungal activity. Furthermore, Cercosporamide and a beta-1,3-glucan synthase inhibitor echinocandin analog, by targeting two different key components of the cell wall biosynthesis pathway, are highly synergistic in their antifungal activities. The synergistic antifungal activity between Pkc1 kinase and beta-1,3-glucan synthase inhibitors points to a potential highly effective combination therapy to treat fungal infections.


