Nexturastat AHDAC6 inhibitor,highly potent and selective CAS# 1403783-31-2 |
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- Entinostat (MS-275,SNDX-275)
Catalog No.:BCC3595
CAS No.:209783-80-2
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- Panobinostat (LBH589)
Catalog No.:BCC3601
CAS No.:404950-80-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1403783-31-2 | SDF | Download SDF |
| PubChem ID | 71462653 | Appearance | Powder |
| Formula | C19 H23 N3 O3 | M.Wt | 341.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 56 mg/mL (164.03 mM) *"≥" means soluble, but saturation unknown. | ||
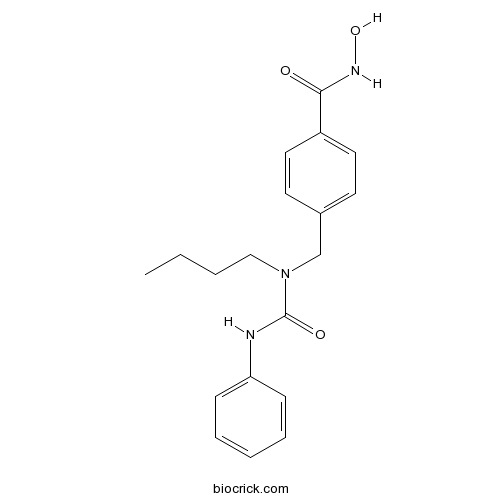
| Chemical Name | 4-[[butyl(phenylcarbamoyl)amino]methyl]-N-hydroxybenzamide | ||
| SMILES | CCCCN(CC1=CC=C(C=C1)C(=O)NO)C(=O)NC2=CC=CC=C2 | ||
| Standard InChIKey | JZWXMCPARMXZQV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H23N3O3/c1-2-3-13-22(19(24)20-17-7-5-4-6-8-17)14-15-9-11-16(12-10-15)18(23)21-25/h4-12,25H,2-3,13-14H2,1H3,(H,20,24)(H,21,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nexturastat A is a highly potent and selective inhibitor of histone deacetylase 6 (HDAC6) with IC50 value of 5 nM. | |||||
| Targets | HDAC6 | |||||
| IC50 | 5 nM | |||||

Nexturastat A Dilution Calculator

Nexturastat A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9291 mL | 14.6456 mL | 29.2912 mL | 58.5823 mL | 73.2279 mL |
| 5 mM | 0.5858 mL | 2.9291 mL | 5.8582 mL | 11.7165 mL | 14.6456 mL |
| 10 mM | 0.2929 mL | 1.4646 mL | 2.9291 mL | 5.8582 mL | 7.3228 mL |
| 50 mM | 0.0586 mL | 0.2929 mL | 0.5858 mL | 1.1716 mL | 1.4646 mL |
| 100 mM | 0.0293 mL | 0.1465 mL | 0.2929 mL | 0.5858 mL | 0.7323 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nexturastat A is a selective inhibitor of histone deacetylase 6 (HDAC6) with IC50 value of 5.2 nM [1].
Nexturastat A is a HDAC inhibitor. It was developed by structural modification of aryl urea HDACIs. The inhibitory activity of Nexturastat A is most potent against HDAC6 with IC50 value of 5.2 nM. Besides that, Nexturastat A also shows inhibition of other HDACs with IC50 values of 3.02, 6.92, 6.68, 9.39, 11.7, 4.46, 0.954, 6.72, 7.57 and 5.14 μM for HDAC1, 2, 3, 4, 5, 7, 8, 9, 10 and 11, respectively. Nexturastat A has been shown to suppress cell proliferation and promote apoptosis in B16 murine melanoma cells [1, 2].
References:
[1] Kalin J H, Bergman J A. Development and therapeutic implications of selective histone deacetylase 6 inhibitors. Journal of medicinal chemistry, 2013, 56(16): 6297-6313.
[2] Zhang L, Han Y, Jiang Q, et al. Trend of Histone Deacetylase Inhibitors in Cancer Therapy: Isoform Selectivity or Multitargeted Strategy. Medicinal research reviews, 2014.
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
- Heteroclitin D
Catalog No.:BCN8166
CAS No.:140369-76-2
- Macranthoidin A
Catalog No.:BCN2808
CAS No.:140360-29-8
- EPZ-6438
Catalog No.:BCC3634
CAS No.:1403254-99-8
- Squalene-2,3-diol
Catalog No.:BCN6220
CAS No.:14031-37-9
- Chetomin
Catalog No.:BCC2432
CAS No.:1403-36-7
- NLG919
Catalog No.:BCC2325
CAS No.:1402836-58-1
- N-Methyllindcarpine
Catalog No.:BCN6218
CAS No.:14028-97-8
- Amoxapine
Catalog No.:BCC4624
CAS No.:14028-44-5
- Boc-Lys(2-Cl-Z)-ol
Catalog No.:BCC2581
CAS No.:14028-05-8
- TUG-770
Catalog No.:BCC2018
CAS No.:1402601-82-4
- Eupalinolide I
Catalog No.:BCN7367
CAS No.:1402067-84-8
- 6'-O-Cinnamoyl-8-epikingisidic acid
Catalog No.:BCN7059
CAS No.:1403984-03-1
- Vancomycin hydrochloride
Catalog No.:BCC4232
CAS No.:1404-93-9
- RSVA 405
Catalog No.:BCC8016
CAS No.:140405-36-3
- CCT244747
Catalog No.:BCC6423
CAS No.:1404095-34-6
- ON 146040
Catalog No.:BCC8058
CAS No.:1404231-34-0
- 7-Methoxycoumarin-4-acetyl-P-L-G-L-β-(2,4-dinitrophenylamino)A-R amide
Catalog No.:BCC1086
CAS No.:140430-53-1
- Fmoc-Dap(Dnp)-OH
Catalog No.:BCC2666
CAS No.:140430-54-2
- ML 281
Catalog No.:BCC6317
CAS No.:1404437-62-2
- GSK 2830371
Catalog No.:BCC4179
CAS No.:1404456-53-6
- 11-Hydroxyjasmonic acid
Catalog No.:BCN6221
CAS No.:140447-14-9
- Ergosterol peroxide glucoside
Catalog No.:BCN6222
CAS No.:140447-22-9
- Heteroclitin C
Catalog No.:BCN3632
CAS No.:140460-42-0
The selective HDAC6 inhibitor Nexturastat A induces apoptosis, overcomes drug resistance and inhibits tumor growth in multiple myeloma.[Pubmed:30782785]
Biosci Rep. 2019 Mar 22;39(3). pii: BSR20181916.
Multiple myeloma (MM) is a hematological malignancy of plasma cells that produce a monoclonal immunoglobulin protein. Despite significant advances in the treatment of MM, challenges such as resistance to therapy remain. Currently, inhibition of histone deacetylases (HDACs) is emerging as a potential method for treating cancers. Numerous HDAC inhibitors are being studied for the use in monotherapy or in conjunction with other agents for MM. In the present study, we investigated the anti-myeloma effect of Nexturastat A (NexA), a novel selective HDAC6 inhibitor. We found that NexA impaired MM cells viability in a dose- and time-dependent manner. NexA also provoked a cell cycle arrest at the G1 phase in MM cells. Furthermore, NexA promoted apoptosis of MM cells via transcriptional activation of the p21 promoter, which may through its ability to up-regulate the H3Ac and H4Ac levels. Additionally, NexA could overcome bortezomib (BTZ) resistance in MM cells, and NexA in combination with BTZ had stronger efficacy. We also confirmed that NexA inhibited tumor growth in murine xenograft models of MM. These interesting findings provided the rationale for the future advancement of this novel HDAC6 inhibitor as a potential therapeutic anti-myeloma agent.


