Entinostat (MS-275,SNDX-275)HDAC1 and HDAC3 inhibitor CAS# 209783-80-2 |
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
- Romidepsin (FK228, depsipeptide)
Catalog No.:BCC3597
CAS No.:128517-07-7
- Pyroxamide
Catalog No.:BCC2424
CAS No.:382180-17-8
- SBHA
Catalog No.:BCC2425
CAS No.:38937-66-5
- KD 5170
Catalog No.:BCC2420
CAS No.:940943-37-3
- Valproic acid
Catalog No.:BCC4260
CAS No.:99-66-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 209783-80-2 | SDF | Download SDF |
| PubChem ID | 4261 | Appearance | Powder |
| Formula | C21H20N4O3 | M.Wt | 376.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | MS-275; SNDX-275 | ||
| Solubility | DMSO : 75 mg/mL (199.25 mM; Need ultrasonic) | ||
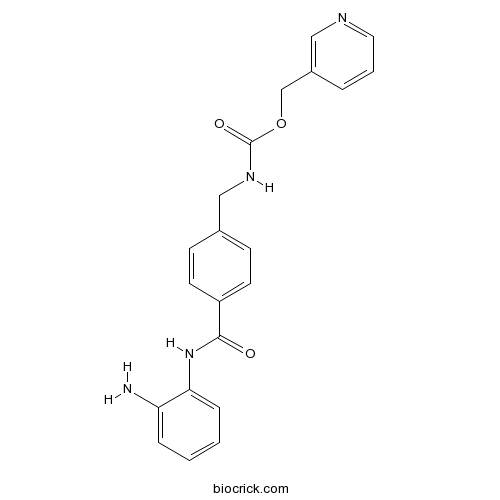
| Chemical Name | pyridin-3-ylmethyl N-[[4-[(2-aminophenyl)carbamoyl]phenyl]methyl]carbamate | ||
| SMILES | C1=CC=C(C(=C1)N)NC(=O)C2=CC=C(C=C2)CNC(=O)OCC3=CN=CC=C3 | ||
| Standard InChIKey | INVTYAOGFAGBOE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Entinostat (MS-275) is a strong inhibitor of HDAC1 and HDAC3 with IC50 of 0.51 μM and 1.7 μM, compared with HDACs 4, 6, 8, and 10. | |||||
| Targets | HDAC1 | HDAC3 | ||||
| IC50 | 0.51 μM | 1.7 μM | ||||
| Cell experiment:[1] | |
| Cell lines | Y79, Weri-Rb1, and Y79-LUC human RB cell lines, and Rb143 primary human RB cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | Dependent on situations |
| Applications | TSA, SAHA, and MS-275 dose dependently reduced RB cell survival. TSA and MS-275 showed additive growth-inhibitory effects in combination with carboplatin, etoposide, or vincristine. TSA and MS-275 increased caspase-3/7 activity. MS-275 increased Annexin V membrane translocation and induced G1arrest. Cytotoxicity of MS-275 was dependent on increased reactive oxygen species levels and was reversed by antioxidant pretreatment. |
| Animal experiment:[1] | |
| Animal models | LHh-Tag transgenic murine model and a rat Y79-LUC ocular xenograft model |
| Dosage form | LHh-Tag mice were treated every other day for 21 d with 20 mg/kg MS-275; Y79-LUC ocular xenografts mice were treated every other day for 13 d with 20 mg/kg MS-275. |
| Application | Intraocular administration of 1μl of 10 μM MS-275 did not alter ocular tissue morphology. Increased acetyl-histone levels confirmed MS-275 delivery to retinal tissue after systemic administration. MS-275 significantly reduced tumor burden in both mouse and rat models of RB. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| Phase I experiment:[2] | |
| Target | Patients with solid tumours. |
| Dosage form | Patients with advanced solid tumours were treated with entinostat orally once weekly and with CRA orally twice daily for 3 weeks in every 4 weeks. The starting dose for entinostat was 4 mg/m2 with a fixed dose of CRA at 1 mg/kg per day. Entinostat dose was escalated by 1 mg/m2 increments. |
| Application | A total of 19 patients were enrolled. The maximum tolerated dose (MTD) was exceeded at the entinostat 5 mg/m2 dose level (G3 hyponatremia, neutropenia, and anaemia). Fatigue (G1 or G2) was a common side effect. Entinostat exhibited substantial variability in clearance (147%) and exposure. The combination of entinostat with CRA was reasonably well tolerated. The recommended phase II doses are entinostat 4 mg/m2 once weekly and CRA 1 mg/kg per day. Although no tumour responses were seen, further evaluation of this combination is warranted. |
| References: 1. Dalgard CL, Van Quill KR, O'Brien JM. Evaluation of the in vitro and in vivo antitumor activity of histone deacetylase inhibitors for the therapy of retinoblastoma. Clin Cancer Res. 2008 May 15;14(10):3113-23. 2. Pili R1, Salumbides B, Zhao M et al. Phase I study of the histone deacetylase inhibitor entinostat in combination with 13-cis retinoic acid in patients with solid tumours. Br J Cancer. 2012 Jan 3;106(1):77-84. | |

Entinostat (MS-275,SNDX-275) Dilution Calculator

Entinostat (MS-275,SNDX-275) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6567 mL | 13.2837 mL | 26.5675 mL | 53.135 mL | 66.4187 mL |
| 5 mM | 0.5313 mL | 2.6567 mL | 5.3135 mL | 10.627 mL | 13.2837 mL |
| 10 mM | 0.2657 mL | 1.3284 mL | 2.6567 mL | 5.3135 mL | 6.6419 mL |
| 50 mM | 0.0531 mL | 0.2657 mL | 0.5313 mL | 1.0627 mL | 1.3284 mL |
| 100 mM | 0.0266 mL | 0.1328 mL | 0.2657 mL | 0.5313 mL | 0.6642 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Entinostat (also known as MS-275 or SNDX-275), a derivative of 2-aminophenyl benzamides, is a potent and orally-available inhibitor of histone deacetylases (HDACs), a family of enzymes associated with a variety of well-characterized cellular oncogenes and tumor suppressors, that potently inhibits class I HDACs, including HDAC1, HDAC3 and HDAC8, with values of 50% inhibition concentration IC50 of 0.368 μM, 0.501 μM and 63.4 μM respectively. Entinostat has been widely investigated for the treatment of cancer, in which results have shown the in vitro anti-proliferative activity of entinostat in a wide range of human cancer cell lines, including breast, colon, lung, myeloma, ovary, pancreas, prostate and leukemia.
Reference
Hess-Stumpp H, Bracker TU, Henderson D, Politz O. MS-275, a potent orally available inhibitor of histone deacetylases--the development of an anticancer agent. Int J Biochem Cell Biol. 2007;39(7-8):1388-405. Epub 2007 Feb 16.
Hess-Stumpp H. Histone deacetylase inhibitors and cancer: from cell biology to the clinic. Eur J Cell Biol. 2005 Mar;84(2-3):109-21.
- Sodium Aescinate
Catalog No.:BCN6266
CAS No.:20977-05-3
- CART (55-102) (rat)
Catalog No.:BCC6006
CAS No.:209615-79-2
- Dihydroisotanshinone I
Catalog No.:BCN2308
CAS No.:20958-18-3
- Isotanshinone I
Catalog No.:BCN2500
CAS No.:20958-17-2
- Isotanshinone IIA
Catalog No.:BCN2501
CAS No.:20958-15-0
- SB 271046 hydrochloride
Catalog No.:BCC1924
CAS No.:209481-24-3
- SB271046
Catalog No.:BCC5057
CAS No.:209481-20-9
- 1-(2-Amino-5-chlorophenyl)-1-(trifluoromethyl)-3-cyclopropyl-2-propyn-1-ol
Catalog No.:BCC8404
CAS No.:209414-27-7
- VX-745
Catalog No.:BCC3966
CAS No.:209410-46-8
- Platycoside A
Catalog No.:BCN3241
CAS No.:209404-00-2
- Beta-mangostin
Catalog No.:BCN1213
CAS No.:20931-37-7
- Neocryptomerin
Catalog No.:BCN8023
CAS No.:20931-36-6
- AR-M 1000390 hydrochloride
Catalog No.:BCC6143
CAS No.:209808-47-9
- Tafluprost
Catalog No.:BCC5270
CAS No.:209860-87-7
- Resibufagin
Catalog No.:BCN8230
CAS No.:20987-24-0
- H-Phenylglycinol
Catalog No.:BCC2713
CAS No.:20989-17-7
- Androst-5-ene-3β,17β-diol 3,17-diacetate
Catalog No.:BCC8823
CAS No.:2099-26-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- LY-411575
Catalog No.:BCC2101
CAS No.:209984-57-6
- LY-411575 isomer 1
Catalog No.:BCC5443
CAS No.:209984-58-7
- LY-900009
Catalog No.:BCC2103
CAS No.:209984-68-9
- Erythbidin A
Catalog No.:BCN6859
CAS No.:210050-83-2
- Nilgirine
Catalog No.:BCN2100
CAS No.:21009-05-2
- Jatrophane I
Catalog No.:BCN7658
CAS No.:210108-85-3
Immunomodulation by Entinostat in Renal Cell Carcinoma Patients Receiving High-Dose Interleukin 2: A Multicenter, Single-Arm, Phase I/II Trial (NCI-CTEP#7870).[Pubmed:28939740]
Clin Cancer Res. 2017 Dec 1;23(23):7199-7208.
Purpose: On the basis of preclinical data suggesting that the class I selective HDAC inhibitor entinostat exerts a synergistic antitumor effect in combination with high-dose IL2 in a renal cell carcinoma model by downregulating Foxp3 expression and function of regulatory T cells (Treg), we conducted a phase I/II clinical study with entinostat and high-dose IL2 in patients with metastatic clear cell renal cell carcinoma (ccRCC).Experimental Design: Clear cell histology, no prior treatments, and being sufficiently fit to receive high-dose IL2 were the main eligibility criteria. The phase I portion consisted of two dose levels of entinostat (3 and 5 mg, orally every 14 days) and a fixed standard dose of IL2 (600,000 U/kg i.v.). Each cycle was 85 days. The primary endpoint was objective response rate and toxicity. Secondary endpoints included progression-free survival and overall survival.Results: Forty-seven patients were enrolled. At a median follow-up of 21.9 months, the objective response rate was 37% [95% confidence interval (CI), 22%-53%], the median progression-free survival was 13.8 months (95% CI, 6.0-18.8), and the median overall survival was 65.3 months (95% CI, 52.6.-65.3). The most common grade 3/4 toxicities were hypophosphatemia (16%), lymphopenia (15%), and hypocalcemia (7%), and all were transient. Decreased Tregs were observed following treatment with entinostat, and lower numbers were associated with response (P = 0.03).Conclusions: This trial suggests a promising clinical activity for entinostat in combination with high-dose IL2 in ccRCC patients and provides the first example of an epigenetic agent being rationally combined with immunotherapy. Clin Cancer Res; 23(23); 7199-208. (c)2017 AACR.
Effect of entinostat on NK cell-mediated cytotoxicity against osteosarcoma cells and osteosarcoma lung metastasis.[Pubmed:28919994]
Oncoimmunology. 2017 Jul 11;6(8):e1333214.
There is a crucial need for a new therapeutic approach for osteosarcoma (OS) lung metastasis since this disease remains the main cause of mortality in OS. We previously demonstrated that natural killer (NK) cell therapy has minimal efficacy against OS metastasis. This study determined whether the histone deacetylase inhibitor entinostat could immunosensitize OS cells to NK cell lysis and increases the efficacy of NK cell therapy for OS lung metastasis. Entinostat upregulated ligands for NK cell-activating receptors (major histocompatibility complex [MHC] class I polypeptide-related chain A [MICA] and B [MICB]; UL16 binding proteins 1, 2, 5, and 6; and CD155) on OS cells both in vitro and in vivo and led to more susceptibility to NK cell-mediated cytotoxicity in vitro. Importantly, entinostat did not change NK cell viability, receptor expression, or function within the 24-h treatment. We also demonstrated two potential mechanisms by which entinostat enhanced expression of MICA and MICB on OS cells. Although entinostat upregulated ligands for the NK cell activating receptor on OS lung metastasis, it failed to augment the efficacy of NK cell therapy in our nude mouse human OS lung metastasis model. This can be partly explained by our finding that although the infused NK cells were active and functional and could penetrate into the lungs, they failed to infiltrate into the lung nodules. These challenges regarding cellular immunotherapy against solid tumors may be overcome by combination therapy, such as adding a NK cell-activating cytokine (IL-2 or IL-21).
Targeting triple negative breast cancer with histone deacetylase inhibitors.[Pubmed:28952409]
Expert Opin Investig Drugs. 2017 Nov;26(11):1199-1206.
INTRODUCTION: Triple negative breast cancer (TNBC) is a heterogeneous disease characterized by poor outcomes, higher rates of relapse, lack of biomarkers for rational use of targeted treatments and insensitivity to current available treatments. Histone deacetylase inhibitors (HDACis) perform multiple cytotoxic actions and are emerging as promising multifunctional agents in TNBC. Areas covered: This review focuses on the challenges so far addressed in the targeted treatment of TNBC and explores the various mechanisms by which HDACis control cancer cell growth, tumor progression and metastases. Pivotal preclinical trials on HDACis like panobinostat, vorinostat, and entinostat show that these epigenetic agents exert an anti-proliferative effect on TNBC cells and control tumor growth by multiple mechanisms of action, including apoptosis and regulation of the epithelial to mesenchimal transition (EMT). Combination studies have reported the synergism of HDACis with other anticancer agents. Expert opinion: In recent years, treatment of TNBC has recorded a high number of failures in the development of targeted agents. HDACis alone or in combination strategies show promising activity in TNBC and could have implications for the future targeted treatment of TNBC patients. Future research should identify which agent synergizes better with HDACis and which patient will benefit more from these epigenetic agents.
Histone Deacetylases as New Therapeutic Targets in Triple-negative Breast Cancer: Progress and Promises.[Pubmed:28870998]
Cancer Genomics Proteomics. 2017 Sep-Oct;14(5):299-313.
Triple-negative breast cancer (TNBC) lacks expression of estrogen receptor (ER), progesterone receptor (PR) and HER2 gene. It comprises approximately 15-20% of breast cancers (BCs). Unfortunately, TNBC's treatment continues to be a clinical problem because of its relatively poor prognosis, its aggressiveness and the lack of targeted therapies, leaving chemotherapy as the mainstay of treatment. It is essential to find new therapies against TNBC, in order to surpass the resistance and the invasiveness of already existing therapies. Given the fact that epigenetic processes control both the initiation and progression of TNBC, there is an increasing interest in the mechanisms, molecules and signaling pathways that participate at the epigenetic modulation of genes expressed in carcinogenesis. The acetylation of histone proteins provokes the transcription of genes involved in cell growth, and the expression of histone deacetylases (HDACs) is frequently up-regulated in many malignancies. Unfortunately, in the field of BC, HDAC inhibitors have shown limited effect as single agents. Nevertheless, their use in combination with kinase inhibitors, autophagy inhibitors, ionizing radiation, or two HDAC inhibitors together is currently being evaluated. HDAC inhibitors such as suberoylanilidehydroxamic acid (SAHA), sodium butyrate, mocetinostat, panobinostat, entinostat, YCW1 and N-(2-hydroxyphenyl)-2-propylpentanamide have shown promising therapeutic outcomes against TNBC, especially when they are used in combination with other anticancer agents. More studies concerning HDAC inhibitors in breast carcinomas along with a more accurate understanding of the TNBC's pathobiology are required for the possible identification of new therapeutic strategies.
Use of a genome-wide haploid genetic screen to identify treatment predicting factors: a proof-of-principle study in pancreatic cancer.[Pubmed:28969017]
Oncotarget. 2017 Jun 29;8(38):63635-63645.
The ability to develop a comprehensive panel of treatment predicting factors would significantly improve our ability to stratify patients for cytotoxic or targeted therapies, and prevent patients receiving ineffective treatments. We have investigated if a recently developed genome-wide haploid genetic screen can be used to reveal the critical mediators of response to anticancer therapy. Pancreatic cancer is known to be highly resistant to systemic therapy. Recently epigenetic changes have been shown to be a key determinant in the maintenance of subpopulations of cancer cells with high-level resistance to cytotoxic therapy. We show that in human pancreatic cancer cell lines, treatment with the potent class I histone deacetylase inhibitor, entinostat, synergistically enhances gemcitabine-induced inhibition of cell proliferation and apoptosis. Using a genome-wide haploid genetic screen, we identified deoxycytidine kinase (DCK) as one of the genes with the highest degree of insertional enrichment following treatment with gemcitabine and entinostat; DCK is already known to be the rate-limiting activating enzyme for gemcitabine. Immunoblotting confirmed loss of DCK protein expression in the resistant KBM7 cells. CRISPR/Cas-9 inactivation of DCK in pancreatic cancer cell lines resulted in resistance to gemcitabine alone and in combination with entinostat. We have identified gemcitabine and entinostat as a potential new combination therapy in pancreatic cancer, and in this proof-of-principle study we have demonstrated that a recently developed haploid genetic screen can be used as a novel approach to identify the critical genes that determine treatment response.


