Isotanshinone ICAS# 20958-17-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20958-17-2 | SDF | Download SDF |
| PubChem ID | 623940 | Appearance | Red powder |
| Formula | C18H12O3 | M.Wt | 276.29 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
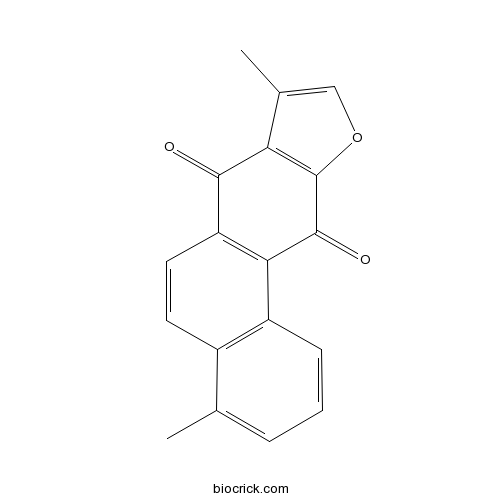
| Chemical Name | 4,8-dimethylnaphtho[2,1-f][1]benzofuran-7,11-dione | ||
| SMILES | CC1=CC=CC2=C1C=CC3=C2C(=O)C4=C(C3=O)C(=CO4)C | ||
| Standard InChIKey | XYKZSUXWBGUGQV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H12O3/c1-9-4-3-5-12-11(9)6-7-13-15(12)17(20)18-14(16(13)19)10(2)8-21-18/h3-8H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Isotanshinone I ,neotanshinone A and cryptotanshinone have different effects on inhibiting proteic activity of P38 and NF-κB signaling pathway,and this effect may be one of main mechanisms of inhibitive inflammation reaction by salvia miltiorrhiza bunge in liver. |
| Targets | p38MAPK | NF-kB | p65 |

Isotanshinone I Dilution Calculator

Isotanshinone I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6194 mL | 18.0969 mL | 36.1939 mL | 72.3877 mL | 90.4846 mL |
| 5 mM | 0.7239 mL | 3.6194 mL | 7.2388 mL | 14.4775 mL | 18.0969 mL |
| 10 mM | 0.3619 mL | 1.8097 mL | 3.6194 mL | 7.2388 mL | 9.0485 mL |
| 50 mM | 0.0724 mL | 0.3619 mL | 0.7239 mL | 1.4478 mL | 1.8097 mL |
| 100 mM | 0.0362 mL | 0.181 mL | 0.3619 mL | 0.7239 mL | 0.9048 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isotanshinone IIA
Catalog No.:BCN2501
CAS No.:20958-15-0
- SB 271046 hydrochloride
Catalog No.:BCC1924
CAS No.:209481-24-3
- SB271046
Catalog No.:BCC5057
CAS No.:209481-20-9
- 1-(2-Amino-5-chlorophenyl)-1-(trifluoromethyl)-3-cyclopropyl-2-propyn-1-ol
Catalog No.:BCC8404
CAS No.:209414-27-7
- VX-745
Catalog No.:BCC3966
CAS No.:209410-46-8
- Platycoside A
Catalog No.:BCN3241
CAS No.:209404-00-2
- Beta-mangostin
Catalog No.:BCN1213
CAS No.:20931-37-7
- Neocryptomerin
Catalog No.:BCN8023
CAS No.:20931-36-6
- Fmoc- ß-HoAsp(OtBu)-OH
Catalog No.:BCC3230
CAS No.:209252-17-5
- Entecavir Hydrate
Catalog No.:BCC1109
CAS No.:209216-23-9
- Obtucarbamate B
Catalog No.:BCN3937
CAS No.:20913-18-2
- Mangochinine
Catalog No.:BCN4913
CAS No.:209115-67-3
- Dihydroisotanshinone I
Catalog No.:BCN2308
CAS No.:20958-18-3
- CART (55-102) (rat)
Catalog No.:BCC6006
CAS No.:209615-79-2
- Sodium Aescinate
Catalog No.:BCN6266
CAS No.:20977-05-3
- Entinostat (MS-275,SNDX-275)
Catalog No.:BCC3595
CAS No.:209783-80-2
- AR-M 1000390 hydrochloride
Catalog No.:BCC6143
CAS No.:209808-47-9
- Tafluprost
Catalog No.:BCC5270
CAS No.:209860-87-7
- Resibufagin
Catalog No.:BCN8230
CAS No.:20987-24-0
- H-Phenylglycinol
Catalog No.:BCC2713
CAS No.:20989-17-7
- Androst-5-ene-3β,17β-diol 3,17-diacetate
Catalog No.:BCC8823
CAS No.:2099-26-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- LY-411575
Catalog No.:BCC2101
CAS No.:209984-57-6
- LY-411575 isomer 1
Catalog No.:BCC5443
CAS No.:209984-58-7
Hepatoprotective Effect of San-Cao Granule on Con A-Induced Liver Injury in Mice and Mechanisms of Action Exploration.[Pubmed:29946260]
Front Pharmacol. 2018 Jun 12;9:624.
Objective: San-Cao granule (SCG), a traditional Chinese herb formula, has been used for treating autoimmune hepatitis (AIH) in our clinics for a long time. However, its active ingredients and mechanisms of action were still unknown due to its complicated chemical compositions. In the present study, the pharmacological study of SCG on acute liver injury induced by Concanavalin A (Con A) was performed to provide a scientific evidence for SCG against liver injury. Methods: In order to screen active components and predicate mechanisms of action, an "ingredients-target-disease" interaction network was constructed by network pharmacology. Then, the pharmacological study was performed to evaluate the therapeutic effect and the underlying mechanisms of SCG on Con A-induced liver injury in mice. Results: This research demonstrated the pharmacological effect of SCG on Con A-induced liver injury, which was through improving the liver function, relieving the pathological changes of liver tissue, decreasing the level of pro-inflammatory cytokines, and thus balancing the pro- and anti-inflammatory cytokines. And the anti-inflammatory of SCG may advantage over the ursodeoxycholic acid (UDCA). Network pharmacology analysis revealed that the pharmacological effect of SCG might be related to its active ingredients of taraxanthin, dihydrotanshinone I, Isotanshinone I, gamma-sitosterol, 3beta-acetyl-20,25-epoxydammarane-24alpha, and delta-7-stigmastenol. The hepatoprotective effect of SCG was reflected by suppressing Con A-induced apoptosis which was mediated by TRAIL and FASL. Conclusion: The combination of network pharmacology and experimental data has revealed the anti-apoptotic effect of SCG against Con A-induced liver injury.
Selective in vitro and in silico butyrylcholinesterase inhibitory activity of diterpenes and rosmarinic acid isolated from Perovskia atriplicifolia Benth. and Salvia glutinosa L.[Pubmed:27817931]
Phytochemistry. 2017 Jan;133:33-44.
Cholinesterase inhibition is one of the most treatment strategies against Alzheimer's disease (AD) where metal accumulation is also strongly associated with pathology of the disease. In the current study, we assessed inhibitory effect against acetyl- (AChE) and butyrylcholinesterase (BChE) and metal-chelating capacity of twelve diterpenes: arucadiol, miltirone, tanshinone IIa, 1-oxomiltirone, cryptotanshinone, 1,2-didehydromiltirone, 1,2-didehydrotanshinone IIa, 1beta-hydroxycryptotanshinone, 15,16-dihydrotanshinone, tanshinone I, Isotanshinone II, 1(S)-hydroxytanshinone IIa, and rosmarinic acid, isolated from Perovskia atriplicifolia and Salvia glutinosa. The compounds were tested at 10 mug/mL using ELISA microtiter assays against AChE and BChE. QSAR and molecular docking studies have been also performed on the active compounds. All of the compounds showed higher [e.g., IC50 = 1.12 +/- 0.07 mug/mL for 1,2-didehydromiltirone, IC50 = 1.15 +/- 0.07 mug/mL for cryptotanshinone, IC50 = 1.20 +/- 0.03 mug/mL for arucadiol, etc.)] or closer [1,2-didehydrotanshinone IIa (IC50 = 5.98 +/- 0.49 mug/mL) and 1(S)-hydroxytanshinone IIa (IC50 = 5.71 +/- 0.27 mug/mL)] inhibition against BChE as compared to that of galanthamine (IC50 = 12.56 +/- 0.37 mug/mL), whereas only 15,16-dihydrotanshinone moderately inhibited AChE (65.17 +/- 1.39%). 1,2-Didehydrotanshinone IIa (48.94 +/- 0.26%) and 1(S)-hydroxytanshinone IIa (47.18 +/- 5.10%) possessed the highest metal-chelation capacity. The present study affords an evidence for the fact that selective BChE inhibitors should be further investigated as promising candidate molecules for AD therapy.
Quinone derivatives by chemical transformations of 16-hydroxycarnosol from Salvia species.[Pubmed:16327182]
Chem Pharm Bull (Tokyo). 2005 Dec;53(12):1524-9.
The known diterpenes 12,16-epoxycarnosol (2), Isotanshinone II (6), and (+)-neocryptotanshinone (8) were obtained by partial synthesis from 16-hydroxycarnosol (1), a C-16 hydroxylated abietatriene diterpene isolated in relative abundance from the aerial part of Salvia mellifera GREENE. The physical and spectroscopic data of these semisynthetic diterpenes were identical to those given for the natural ones in the literature. These abietane diterpenes have very interesting biological activities and the semisynthetic approach described here represents an alternative to obtain them from other major diterpenes isolated from Salvia species. Additionally, seven new semisynthetic diterpene analogues, 11,14-dioxo-12,16-epoxy-8,12-abietadien-20,7beta-olide (3), 11,14-dioxo-12,16-epoxy-8,12,15(16)-abietatrien-20,7beta-olide (4), 15,16-didehydro-12,16-epoxycarnosol (5), 1-oxoIsotanshinone II (7), 16-hydroxycolumbaridione (9), 12,16-diacetoxycolumbaridione (10), and 14-methoxy-12,16-epoxycarnosol (13), were obtained from 1. The structures of the new compounds were established based on their spectroscopic data.
PTP1B inhibitory effect of abietane diterpenes isolated from Salvia miltiorrhiza.[Pubmed:16141564]
Biol Pharm Bull. 2005 Sep;28(9):1795-7.
Protein tyrosine phosphatase 1B (PTP1B) acts as a negative regulator of insulin signaling, and selective inhibition of PTP1B has served as a potential drug target for the treatment of type 2 diabetes. In the course of screening for PTP1B inhibitory natural products, the MeOH extract of the dried root of Salvia miltiorrhiza BUNGE (Labiatae) was found to exhibit significant inhibitory effect. Bioassay-guided fractionation and purification afforded three related abietane-type diterpene metabolites 1-3. Compounds 1-3 were identified as Isotanshinone IIA (1), dihydroIsotanshinone I (2), and isocryptotanshinone (3) mainly by analysis of NMR and MS data. Compounds 1-3 non-competitively inhibited PTP1B activity with 50% inhibitory concentration values of 11.4+/-0.6 microM, 22.4+/-0.6 microM and 56.1+/-6.3 microM, respectively.
Isolation and bioactivity of new tanshinones.[Pubmed:3655791]
J Nat Prod. 1987 Mar-Apr;50(2):157-60.
Two new diterpenoids, designated neocryptotanshinone and Isotanshinone IIB, have been isolated from "Tan-Shen," the root of Salvia miltiorrhiza, together with a known compound, danshexinkun A. Their structures are established by spectral and physical data. Isotanshinone IIB exhibits significant inhibitory activity in vitro on ADP- and collagen-induced aggregation.


