VX-745P38α inhibitor,highly potent and selective CAS# 209410-46-8 |
- AL 8697
Catalog No.:BCC8037
CAS No.:1057394-06-5
- Skepinone-L
Catalog No.:BCC1953
CAS No.:1221485-83-1
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- TAK-715
Catalog No.:BCC3968
CAS No.:303162-79-0
- SB 203580 hydrochloride
Catalog No.:BCC4293
CAS No.:869185-85-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 209410-46-8 | SDF | Download SDF |
| PubChem ID | 3038525 | Appearance | Powder |
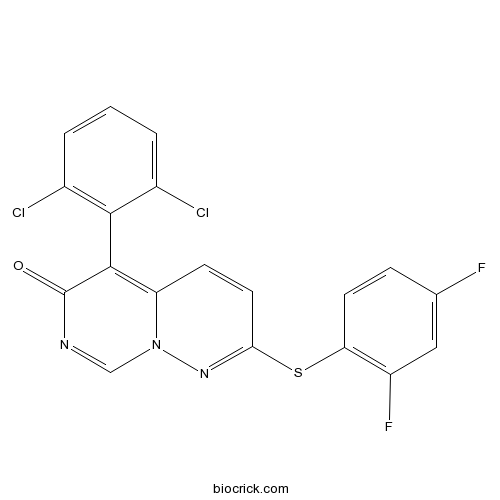
| Formula | C19H9Cl2F2N3OS | M.Wt | 436.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Neflamapimod | ||
| Solubility | DMSO : 13.08 mg/mL (29.98 mM; Need ultrasonic) | ||
| Chemical Name | 5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfanylpyrimido[1,6-b]pyridazin-6-one | ||
| SMILES | C1=CC(=C(C(=C1)Cl)C2=C3C=CC(=NN3C=NC2=O)SC4=C(C=C(C=C4)F)F)Cl | ||
| Standard InChIKey | VEPKQEUBKLEPRA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H9Cl2F2N3OS/c20-11-2-1-3-12(21)17(11)18-14-5-7-16(25-26(14)9-24-19(18)27)28-15-6-4-10(22)8-13(15)23/h1-9H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly potent and selective p38α inhibitor (IC50 = 10 nM). Also blocks TNFα production in LPS-stimulated HWB in vitro (IC50 = 177 nM). Displays 1000-fold selectivity over closely related kinases, including ERK1, MK2 and JNK1-3. |

VX-745 Dilution Calculator

VX-745 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2922 mL | 11.4608 mL | 22.9216 mL | 45.8432 mL | 57.304 mL |
| 5 mM | 0.4584 mL | 2.2922 mL | 4.5843 mL | 9.1686 mL | 11.4608 mL |
| 10 mM | 0.2292 mL | 1.1461 mL | 2.2922 mL | 4.5843 mL | 5.7304 mL |
| 50 mM | 0.0458 mL | 0.2292 mL | 0.4584 mL | 0.9169 mL | 1.1461 mL |
| 100 mM | 0.0229 mL | 0.1146 mL | 0.2292 mL | 0.4584 mL | 0.573 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
VX-745 is a first-generation inhibitor of p38αMAPK with IC50 value of 1.0 μM when tested with Werner syndrome dermal fibroblasts [1].
Mammalian mitogen-activated protein (MAP) kinases are serine /threonine kinases and have 4 subfamilies: ERK, JNK, MAPKp38 and ERK5. MAPK pathway plays a pivotal role in transmitting signals from cell membrane to nucleus and also participates in many intracellular signaling pathways that control a wide spectrum of cellular processes (growth, differentiation, stress responses, cancer progression) [2]. MAPKp38 is originally isolated from lipopolysaccharide-stimulated monocytes. And according to the distribution in tissue, regulation of kinase activation and subsequent phosphorylation of downstream substrates, it is divided into four isoforms p38alpha, p38beta, p38gamma and p38delta [3].
VX-745 is an exquisite kinase selectively inhibit p38αMAPK and is regarded as an anti-inflammatory candidate. When tested with PBMCs or whole blood, VX-745 treatment could inhibit the secretion of IL-1β and TNF-α in vitro [4]. In human dermal fibroblast cells, VX-745 treatment blocked p38 signaling to rescue the aging phenotype in Werner syndrome [5].
References:
[1]. Bagley, M.C., et al., Microwave-assisted Ullmann C-S bond formation: synthesis of the P38alpha MAPK clinical candidate VX-745. J Org Chem, 2009. 74(21): p. 8336-42.
[2]. Dent, P., et al., MAPK pathways in radiation responses. Oncogene, 2003. 22(37): p. 5885-96.
[3]. Dominguez, C., D.A. Powers, and N. Tamayo, p38 MAP kinase inhibitors: many are made, but few are chosen. Curr Opin Drug Discov Devel, 2005. 8(4): p. 421-30.
[4]. Duffy, J.P., et al., The Discovery of VX-745: A Novel and Selective p38alpha Kinase Inhibitor. ACS Med Chem Lett, 2011. 2(10): p. 758-63.
[5]. Bagley, M.C., et al., Gram-scale synthesis of the p38alpha MAPK-inhibitor VX-745 for preclinical studies into Werner syndrome. Future Med Chem, 2010. 2(9): p. 1417-27.
- Platycoside A
Catalog No.:BCN3241
CAS No.:209404-00-2
- Beta-mangostin
Catalog No.:BCN1213
CAS No.:20931-37-7
- Neocryptomerin
Catalog No.:BCN8023
CAS No.:20931-36-6
- Fmoc- ß-HoAsp(OtBu)-OH
Catalog No.:BCC3230
CAS No.:209252-17-5
- Entecavir Hydrate
Catalog No.:BCC1109
CAS No.:209216-23-9
- Obtucarbamate B
Catalog No.:BCN3937
CAS No.:20913-18-2
- Mangochinine
Catalog No.:BCN4913
CAS No.:209115-67-3
- JWH 073
Catalog No.:BCC1674
CAS No.:208987-48-8
- Swertianin
Catalog No.:BCC8258
CAS No.:20882-75-1
- Testosterone benzoate
Catalog No.:BCC9166
CAS No.:2088-71-3
- Saikosaponin D
Catalog No.:BCN1088
CAS No.:20874-52-6
- (1R,1'S,3'R/1R,1'R,3'S)-L-054,264
Catalog No.:BCC7364
CAS No.:208706-12-1
- 1-(2-Amino-5-chlorophenyl)-1-(trifluoromethyl)-3-cyclopropyl-2-propyn-1-ol
Catalog No.:BCC8404
CAS No.:209414-27-7
- SB271046
Catalog No.:BCC5057
CAS No.:209481-20-9
- SB 271046 hydrochloride
Catalog No.:BCC1924
CAS No.:209481-24-3
- Isotanshinone IIA
Catalog No.:BCN2501
CAS No.:20958-15-0
- Isotanshinone I
Catalog No.:BCN2500
CAS No.:20958-17-2
- Dihydroisotanshinone I
Catalog No.:BCN2308
CAS No.:20958-18-3
- CART (55-102) (rat)
Catalog No.:BCC6006
CAS No.:209615-79-2
- Sodium Aescinate
Catalog No.:BCN6266
CAS No.:20977-05-3
- Entinostat (MS-275,SNDX-275)
Catalog No.:BCC3595
CAS No.:209783-80-2
- AR-M 1000390 hydrochloride
Catalog No.:BCC6143
CAS No.:209808-47-9
- Tafluprost
Catalog No.:BCC5270
CAS No.:209860-87-7
- Resibufagin
Catalog No.:BCN8230
CAS No.:20987-24-0
The Discovery of VX-745: A Novel and Selective p38alpha Kinase Inhibitor.[Pubmed:24900264]
ACS Med Chem Lett. 2011 Jul 28;2(10):758-63.
The synthesis of novel, selective, orally active 2,5-disubstituted 6H-pyrimido[1,6-b]pyridazin-6-one p38alpha inhibitors is described. Application of structural information from enzyme-ligand complexes guided the selection of screening compounds, leading to the identification of a novel class of p38alpha inhibitors containing a previously unreported bicyclic heterocycle core. Advancing the SAR of this series led to the eventual discovery of 5-(2,6-dichlorophenyl)-2-(2,4-difluorophenylthio)-6H-pyrimido[1,6-b]pyridazin-6-o ne (VX-745). VX-745 displays excellent enzyme activity and selectivity, has a favorable pharmacokinetic profile, and demonstrates good in vivo activity in models of inflammation.
Microwave-assisted Ullmann C-S bond formation: synthesis of the P38alpha MAPK clinical candidate VX-745.[Pubmed:19778055]
J Org Chem. 2009 Nov 6;74(21):8336-42.
Microwave irradiation promotes the rapid and efficient reaction of a thiophenol and aryl or heteroaryl halide using a copper or palladium catalyst and a range of ligands, depending upon substrate. Of particular utility is the use of copper(I) iodide (5 mol %) and trans-cyclohexane-1,2-diol as ligand under basic conditions and microwave irradiation to give the corresponding sulfide in high yield. This method for C-S bond formation is applied in the four-step synthesis of the clinical candidate VX-745 in 38% overall yield. The inhibitory activity of VX-745 against p38alpha MAPK is confirmed in Werner syndrome dermal fibroblasts at 1.0 microM concentration by immunoblot assay.
Rapid synthesis of VX-745: p38 MAP kinase inhibition in Werner syndrome cells.[Pubmed:17659871]
Bioorg Med Chem Lett. 2007 Sep 15;17(18):5107-10.
The p38 mitogen-activated protein kinase inhibitor VX-745 is prepared rapidly and efficiently in a four-step sequence using a combination of conductive heating and microwave-mediated steps. Its inhibitory activity was confirmed in hTERT immortalized HCA2 and WS dermal fibroblasts at 0.5-1.0 microM concentration by ELISA and immunoblot assay, and displays excellent kinase selectivity over the related stress-activated kinase JNK.
Gram-scale synthesis of the p38alpha MAPK-inhibitor VX-745 for preclinical studies into Werner syndrome.[Pubmed:21426137]
Future Med Chem. 2010 Sep;2(9):1417-27.
BACKGROUND: The ATP-competitive p38alpha MAPK inhibitor VX-745 exhibits an exquisite kinase selectivity profile, is effective in blocking p38 stress signaling in Werner syndrome dermal fibroblasts, has efficacy in clinical trials and may have therapeutic value against Werner syndrome. Previous synthetic routes, however, have only resulted in milligram quantities suitable for cell-based studies, whereas gram quantities would be required for in vivo use. RESULTS & DISCUSSION: Microwave irradiation using a stop-flow monomodal microwave reactor has been found to facilitate scale-up of the synthesis of VX-745. Ullmann-type C-S bond formation using thiophenol, chloropyridazine, copper(I) catalyst and diol ligand proceeds rapidly and efficiently in this apparatus for elaboration to the pyrimido[1,6-b]pyridazinone core of VX-745 on gram scale and with good overall yield. CONCLUSION: This method delivers the p38 inhibitor VX-745 in sufficient quantities for preclinical studies to rescue the aging phenotype in Werner syndrome.


