SwertianinCAS# 20882-75-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20882-75-1 | SDF | Download SDF |
| PubChem ID | 5281661 | Appearance | Powder |
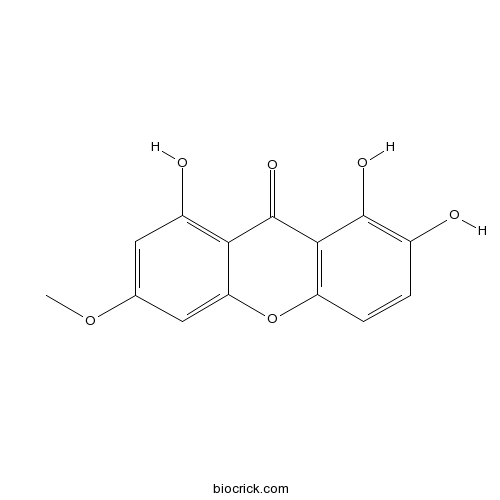
| Formula | C14H10O6 | M.Wt | 274 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,2,8-trihydroxy-6-methoxyxanthen-9-one | ||
| SMILES | COC1=CC(=C2C(=C1)OC3=C(C2=O)C(=C(C=C3)O)O)O | ||
| Standard InChIKey | BDBVOZGRVBXANN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H10O6/c1-19-6-4-8(16)11-10(5-6)20-9-3-2-7(15)13(17)12(9)14(11)18/h2-5,15-17H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Swertianin may be a promising antioxidant. 2. Swertianin has potential anti-inflammatory and antinoceceptive which could be used as drug candidates against inflammation related conditions. 3. Swertianin exhibits significant anti-proliferative activity. |
| Targets | Immunology & Inflammation related | Antifection |

Swertianin Dilution Calculator

Swertianin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6496 mL | 18.2482 mL | 36.4964 mL | 72.9927 mL | 91.2409 mL |
| 5 mM | 0.7299 mL | 3.6496 mL | 7.2993 mL | 14.5985 mL | 18.2482 mL |
| 10 mM | 0.365 mL | 1.8248 mL | 3.6496 mL | 7.2993 mL | 9.1241 mL |
| 50 mM | 0.073 mL | 0.365 mL | 0.7299 mL | 1.4599 mL | 1.8248 mL |
| 100 mM | 0.0365 mL | 0.1825 mL | 0.365 mL | 0.7299 mL | 0.9124 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Testosterone benzoate
Catalog No.:BCC9166
CAS No.:2088-71-3
- Saikosaponin D
Catalog No.:BCN1088
CAS No.:20874-52-6
- (1R,1'S,3'R/1R,1'R,3'S)-L-054,264
Catalog No.:BCC7364
CAS No.:208706-12-1
- Ermanin
Catalog No.:BCN4912
CAS No.:20869-95-8
- Boc-His(Boc)-OH
Catalog No.:BCC3399
CAS No.:20866-46-0
- Berberine
Catalog No.:BCN4911
CAS No.:2086-83-1
- Primulic Acid 2
Catalog No.:BCC8237
CAS No.:208599-88-6
- H-Tle-OH
Catalog No.:BCC2659
CAS No.:20859-02-3
- Micafungin sodium
Catalog No.:BCC1750
CAS No.:208538-73-2
- Protoaescigenin
Catalog No.:BCC8240
CAS No.:20853-07-0
- Stigmastane-3,5,6-triol
Catalog No.:BCN4910
CAS No.:20835-91-0
- Ethyl 4-(rhamnosyloxy)benzylcarbamate
Catalog No.:BCN7635
CAS No.:208346-80-9
- JWH 073
Catalog No.:BCC1674
CAS No.:208987-48-8
- Mangochinine
Catalog No.:BCN4913
CAS No.:209115-67-3
- Obtucarbamate B
Catalog No.:BCN3937
CAS No.:20913-18-2
- Entecavir Hydrate
Catalog No.:BCC1109
CAS No.:209216-23-9
- Fmoc- ß-HoAsp(OtBu)-OH
Catalog No.:BCC3230
CAS No.:209252-17-5
- Neocryptomerin
Catalog No.:BCN8023
CAS No.:20931-36-6
- Beta-mangostin
Catalog No.:BCN1213
CAS No.:20931-37-7
- Platycoside A
Catalog No.:BCN3241
CAS No.:209404-00-2
- VX-745
Catalog No.:BCC3966
CAS No.:209410-46-8
- 1-(2-Amino-5-chlorophenyl)-1-(trifluoromethyl)-3-cyclopropyl-2-propyn-1-ol
Catalog No.:BCC8404
CAS No.:209414-27-7
- SB271046
Catalog No.:BCC5057
CAS No.:209481-20-9
- SB 271046 hydrochloride
Catalog No.:BCC1924
CAS No.:209481-24-3
Evaluation of anticonvulsant, sedative, anxiolytic, and phytochemical profile of the methanol extract from the aerial parts of Swertia corymbosa (Griseb.) wight ex C.B. Clarke.[Pubmed:24877112]
Biomed Res Int. 2014;2014:542385.
The objective of the present study was to evaluate the anxiolytic, antidepressant, and anticonvulsant activity of the methanolic extract of Swertia corymbosa (SCMeOH). After acute toxicity test, oral treatment with SCMeOH at doses of 125, 250, and 500 mg/kg behavioral models of open field, elevated-plus-maze, actophotometer, rotarod, pentylenetetrazole, isoniazid, and maximal electroshock induced seizure models were utilized. In open field test, SCMeOH (125, 250, and 500 mg/kg) (P < 0.01, P < 0.001) increased the number of rearings. However, the number of central motor and ambulation (P < 0.01, P < 0.001) were reduced. Likewise, the number of entries and the time spent in open arm were increased while the number of locomotion was decreased (P < 0.001) in elevated-plus-maze and actophotometer test, respectively. SCMeOH (125-500 mg/kg) protected the mice against the pentylenetetrazole and isoniazid induced convulsions; it causes significant (P < 0.01 and P < 0.001) dose dependent increase in latency of convulsion. Treatment with SCMeOH reduced the duration of the tonic hind limb extension induced by electroshock. Two major compounds such as gentiopicroside and Swertianin were analyzed by HPLC system.
RETRACTED: Anti-diabetic activity of Swertia corymbosa (Griseb.) Wight ex C.B. Clarke aerial parts extract in streptozotocin induced diabetic rats.[Pubmed:24378350]
J Ethnopharmacol. 2014 Feb 12;151(3):1175-1183.
ETHNOPHARMACOLOGICALS RELEVANCE: Swertia corymbosa locally called as Shirattakuchi have a long history of use in Ayurveda herbal preparations in Indian traditional system of medicine. It has been used in folklore medicine for the treatment of diabetes. AIM OF THE STUDY: The present study aimed to investigate the effect of the methanolic extract of Swertia corymbosa (SC) in diabetic and to analyze its chemical composition by HPLC-ESI/MS that may correlate with their pharmacological activities. MATERIALS AND METHODS: The in vitro anti-diabetic activity of the extracts was measured by using alpha-glucosidase and alpha-amylase enzyme inhibitory activity. The methanolic extract of Swertia corymbosa were administered orally (125, 250 and 500 mg/kg, for 28 days) to streptozotocin-induced diabetic rats. Hypoglycemic effects, oral glucose tolerance test, change in body weight and lipid profile, biochemical analysis and histopathological examination were assessed. High-performance liquid chromatography-electrospray ionization/mass spectrometry (HPLC-ESI/MS) method was also developed to analyze the chemical composition. RESULTS: In vitro anti-dabetic study, the methanol extract of SC is found to be a potent inhibitor of alpha-glucosidase and alpha-amylase activity. Oral administration of SC and standard drug for 28 days caused a significant decrease in the concentrations of blood glucose level, total cholesterol (TC), serum triglycerides (TGs), low-density lipoprotein-cholesterol (LDL-C), malondialdehyde (MDA) and significant increase in the concentrations of high density lipoprotein-cholesterol (HDL-C), serum insulin and body weight. Furthermore, activities of antioxidative enzymes, including SOD, GPx, GSH and CAT were enhanced dosed dependently with SC. Histopathological studies of the pancreas showed the regeneration of the beta-cells by extract which were earlier necrosed by streptozotocin. Ten major compounds such as loganic acid (1), swertiamarin (2), sweroside (3), gentiopicroside (4), isovitexin (5), amoroswertin (6), amarogentin (7), gentiacaulein (8), decussatin (9) and Swertianin (10) were analyzed by HPLC-ESI/MS system. CONCLUSIONS: These results demonstrate that SC aerial parts of methanolic extract is an effective anti-diabetic and antioxidant activities which provides the scientific proof for the folklore medicine.
Activity-guided isolation of antioxidant xanthones from Swertia chirayita (Roxb.) H. Karsten (Gentianaceae).[Pubmed:21985644]
Nat Prod Res. 2012;26(18):1682-6.
An activity-guided isolation and purification process was used to identify the DPPH (l,l-diphenyl-2-picrylhydrazyl) radical-scavenging components of Swertia chirayita. A dry, whole plant of S. chirayita was extracted with different solvents and tested for its DPPH radical-scavenging activity. The acetone : water (8 : 2) extract showed the highest total phenolic content (TPC) and DPPH radical-scavenging activity, which was column chromatographed to obtain decussatin, Swertianin, bellidifolin, isobellidifolin, amarogentin, swertianolin and mangiferin as active components. Good correlation was observed between TPC and DPPH scavenging activity among the extracts. The unique structure of xanthones, including the catecholic moiety and the completely conjugated system, enables them to be promising antioxidants.
[Study on chemical constituents from Swertia kauitchensis Franch].[Pubmed:15807240]
Zhong Yao Cai. 2004 Dec;27(12):908-10.
OBJECTIVE: To study the major chemical constituents from the whole herb of Swertia kauitchensis Franch. METHOD: Compounds were separated and purified with silica gel column and microreticular resin, and their structures were elucidated on the basis of spectroscopic evidence (UV, IR, MS, NMR). RESULT: Ten compounds were identified as oleanolic acid (1), beta-sitosterol (2), daucosterol (3), gentiacaulein (4), bellidifolin (5), methylSwertianin (6), Swertianin (7), 1,7-dihydroxy-3,4,8-trimethoxyxanthone (8), swertianolin (9),1,3,5,8-tetrahydroxyxanthone (10). CONCLUSION: All compounds were isolated from Swertia kauitchensis Franch for the first time.


