AL 8697p38α inhibitor,potent and selective CAS# 1057394-06-5 |
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- Perifosine
Catalog No.:BCC3673
CAS No.:157716-52-4
- Everolimus (RAD001)
Catalog No.:BCC3594
CAS No.:159351-69-6
- Temsirolimus
Catalog No.:BCC3678
CAS No.:162635-04-3
- Rapamycin (Sirolimus)
Catalog No.:BCC3592
CAS No.:53123-88-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1057394-06-5 | SDF | Download SDF |
| PubChem ID | 25060093 | Appearance | Powder |
| Formula | C21H21F3N4O | M.Wt | 402.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 50 mM in ethanol | ||
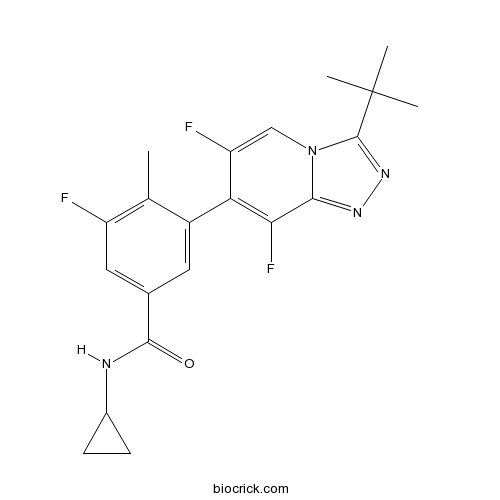
| Chemical Name | 3-(3-tert-butyl-6,8-difluoro-[1,2,4]triazolo[4,3-a]pyridin-7-yl)-N-cyclopropyl-5-fluoro-4-methylbenzamide | ||
| SMILES | CC1=C(C=C(C=C1C2=C(C3=NN=C(N3C=C2F)C(C)(C)C)F)C(=O)NC4CC4)F | ||
| Standard InChIKey | ZVBTZTQYHOXIBC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H21F3N4O/c1-10-13(7-11(8-14(10)22)19(29)25-12-5-6-12)16-15(23)9-28-18(17(16)24)26-27-20(28)21(2,3)4/h7-9,12H,5-6H2,1-4H3,(H,25,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective p38α inhibitor (IC50 = 6 nM). Exhibits 14-fold greater inhibition of p38α compared to p38β; also displays 300-fold selectivity for p38α compared to a panel of 91 kinases. Exhibits anti-inflammatory properties. |

AL 8697 Dilution Calculator

AL 8697 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.485 mL | 12.4251 mL | 24.8503 mL | 49.7006 mL | 62.1257 mL |
| 5 mM | 0.497 mL | 2.485 mL | 4.9701 mL | 9.9401 mL | 12.4251 mL |
| 10 mM | 0.2485 mL | 1.2425 mL | 2.485 mL | 4.9701 mL | 6.2126 mL |
| 50 mM | 0.0497 mL | 0.2485 mL | 0.497 mL | 0.994 mL | 1.2425 mL |
| 100 mM | 0.0249 mL | 0.1243 mL | 0.2485 mL | 0.497 mL | 0.6213 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AL8697 is a selective p38 MAPK(mitogen-activated protein Kinase) inhibitor, which is also named P38 α inhibitor. It has the function of inhibiting the activity of P38 MAPK.[1]
Mitogen activated protein kinases (MAPK) are a group of signaling molecules that appear to play important roles in inflammatory processes. The P38 MAPK is one of the best described MAPK cascades. The p38 MAPK plays a key role in both the synthesis and the signalling of pro-inflammatory cytokines such as TNFa and IL-6 by monocyte/macrophages. The p38 MAPK are involved in the up-regulation of TNF production by murine macrophages.It has high activity in cardiovascular cells under a variety of cellular stresses. In cardiovascular disease, the P38 MAPK signaling pathways are activated.[2,3]
AL8697 function by the inhibition of P38 MAPK. It regulates a variety of cell activities related with P38 MAPK.
In a rat adjuvant-induced arthritis model, AL8697 exhibited a good anti-inflammatory effect and induced leukocytosis and increased total plasma cholesterol, these properties were evidently at 10 mg/kg. In addition, AL8697 partially restored the platelet count. The complex profile for AL8697 in rat AIA (adjuvant-induced arthritis) is not observed in human RA (rheumatoid arthritis).[1]
References:
[1]. Balagué C, Pont M, Prats N, Godessart N. Profiling of dihydroorotate dehydrogenase, p38 and JAK inhibitors in the rat adjuvant-induced arthritis model: a translational study. Br J Pharmacol. 2012 Jun;166(4):1320-32
[2]. Ajizian SJ, English BK, Meals EA.Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J Infect Dis. 1999 Apr;179(4):939-44.
[3]. Bao W, Behm DJ, Nerurkar SS, Ao Z, et al. Effects of p38 MAPK Inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J Cardiovasc Pharmacol. 2007 Jun;49(6):362-8.
- Androstanolone 17-benzoate
Catalog No.:BCC8825
CAS No.:1057-07-4
- AT13148
Catalog No.:BCC5360
CAS No.:1056901-62-2
- CYT387 sulfate salt
Catalog No.:BCC1506
CAS No.:1056636-06-6
- CYT387
Catalog No.:BCC2196
CAS No.:1056634-68-4
- Mizolastine dihydrochloride
Catalog No.:BCC4132
CAS No.:1056596-82-7
- ML 9 hydrochloride
Catalog No.:BCC6644
CAS No.:105637-50-1
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- Fasudil (HA-1077) HCl
Catalog No.:BCC2542
CAS No.:105628-07-7
- Prostephanaberrine
Catalog No.:BCN4736
CAS No.:105608-27-3
- BMY 14802 hydrochloride
Catalog No.:BCC5759
CAS No.:105565-55-7
- Ginsenoside Rh3
Catalog No.:BCN1071
CAS No.:105558-26-7
- Fmoc-Glycinol
Catalog No.:BCC3094
CAS No.:105496-31-9
- Ganoderic acid C6
Catalog No.:BCN3257
CAS No.:105742-76-5
- Methyl ganoderate C6
Catalog No.:BCN3259
CAS No.:105742-81-2
- Fmoc-Ser(tBu)-OPfp
Catalog No.:BCC3545
CAS No.:105751-13-1
- Sitostenone
Catalog No.:BCN5868
CAS No.:1058-61-3
- E-3810
Catalog No.:BCC1541
CAS No.:1058137-23-7
- Nateglinide
Catalog No.:BCC5005
CAS No.:105816-04-4
- Tropisetron Hydrochloride
Catalog No.:BCC4027
CAS No.:105826-92-4
- TSTU
Catalog No.:BCC2828
CAS No.:105832-38-0
- Obtusilin
Catalog No.:BCN2697
CAS No.:105870-59-5
- (tert-Butoxycarbonyl)oxycefcapene pivoxil
Catalog No.:BCC8403
CAS No.:105889-80-3
- Taraxasterol
Catalog No.:BCN5869
CAS No.:1059-14-9
- Doxycycline HCl
Catalog No.:BCC3772
CAS No.:10592-13-9
Novel triazolopyridylbenzamides as potent and selective p38alpha inhibitors.[Pubmed:22521646]
Bioorg Med Chem Lett. 2012 May 15;22(10):3431-6.
A new class of p38alpha inhibitors based on a biaryl-triazolopyridine scaffold was investigated. X-ray crystallographic data of the initial lead compound cocrystallised with p38alpha was crucial in order to uncover a unique binding mode of the inhibitor to the hinge region via a pair of water molecules. Synthesis and SAR was directed towards the improvement of binding affinity, as well as ADME properties for this new class of p38alpha inhibitors and ultimately afforded compounds showing good in vivo efficacy.
Profiling of dihydroorotate dehydrogenase, p38 and JAK inhibitors in the rat adjuvant-induced arthritis model: a translational study.[Pubmed:22229697]
Br J Pharmacol. 2012 Jun;166(4):1320-32.
BACKGROUND AND PURPOSE: Translational animal models are essential in the prediction of the efficacy and side effects of new chemical entities. We have carried out a thorough study of three distinct disease-modifying antirheumatic drugs (DMARDs) in an adjuvant-induced arthritis (AIA) model in the rat and critically appraised the results in the context of the reported clinical experience in rheumatoid arthritis (RA) patients. EXPERIMENTAL APPROACH: Teriflunomide - a dihydroorotate dehydrogenase (DHODH) inhibitor; AL8697 - a selective p38 MAPK inhibitor; and tofacitinib - a Janus kinase (JAK) inhibitor; were selected as representatives of their class and dose-response studies carried out using a therapeutic 10-day administration scheme in arthritic rats. Paw swelling and body weight were periodically monitored, and joint radiology and histology, lymph organ weight and haematological and biochemical parameters evaluated at study completion. KEY RESULTS: All three drugs demonstrated beneficial effects on paw swelling, bone lesions and splenomegalia, with p38 inhibition providing the best anti-inflammatory effect and JAK inhibition the best DMARD effect. Leukopenia, body weight loss and gastrointestinal toxicity were dose-dependently observed with teriflunomide treatment. p38 MAPK inhibition induced leukocytosis and increased total plasma cholesterol. JAK inhibition, normalized platelet, reticulocyte and neutrophil counts, and alanine aminotransferase (ALT) levels while inducing lymphopenia and cholesterolemia. CONCLUSIONS AND IMPLICATIONS: This multiparametric approach can reveal specific drug properties and provide translational information. Whereas the complex profile for p38 inhibition in AIA is not observed in human RA, immunosuppressants such as DHODH and JAK inhibitors show DMARD properties and side effects seen in both AIA and RA.


