BMY 14802 hydrochlorideSigma receptor antagonist CAS# 105565-55-7 |
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 105565-55-7 | SDF | Download SDF |
| PubChem ID | 3086514 | Appearance | Powder |
| Formula | C18H23ClF2N4O | M.Wt | 384.86 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water | ||
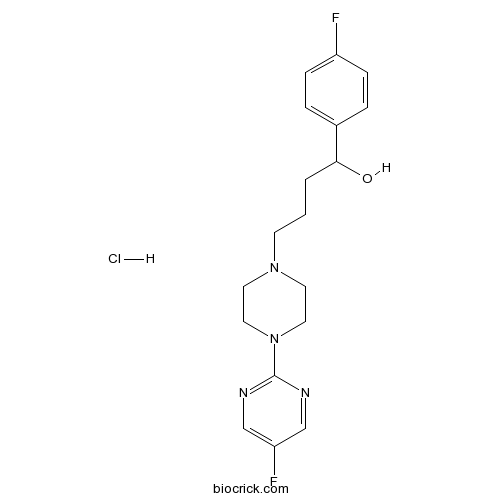
| Chemical Name | 1-(4-fluorophenyl)-4-[4-(5-fluoropyrimidin-2-yl)piperazin-1-yl]butan-1-ol;hydrochloride | ||
| SMILES | C1CN(CCN1CCCC(C2=CC=C(C=C2)F)O)C3=NC=C(C=N3)F.Cl | ||
| Standard InChIKey | NIBVEFRJDFVQLM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H22F2N4O.ClH/c19-15-5-3-14(4-6-15)17(25)2-1-7-23-8-10-24(11-9-23)18-21-12-16(20)13-22-18;/h3-6,12-13,17,25H,1-2,7-11H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent sigma receptor antagonist (IC50 = 112 nM) with modest to weak affinity for 5-HT1A and α1 receptors. Antipsychotic following oral administration and acts via indirect modulation of central dopaminergic systems. |

BMY 14802 hydrochloride Dilution Calculator

BMY 14802 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5983 mL | 12.9917 mL | 25.9835 mL | 51.9669 mL | 64.9587 mL |
| 5 mM | 0.5197 mL | 2.5983 mL | 5.1967 mL | 10.3934 mL | 12.9917 mL |
| 10 mM | 0.2598 mL | 1.2992 mL | 2.5983 mL | 5.1967 mL | 6.4959 mL |
| 50 mM | 0.052 mL | 0.2598 mL | 0.5197 mL | 1.0393 mL | 1.2992 mL |
| 100 mM | 0.026 mL | 0.1299 mL | 0.2598 mL | 0.5197 mL | 0.6496 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 112 nM for (+)-[3H]-3-PPP
The theoretical role of sigma receptors in psychosis has led to the discovery of selective sigma receptor ligands as potential antipsychotic agents. BMY 14802 is a sigma receptor ligand.
In vitro: BMY 14802 has its most potent binding at the sigma binding site, with some degree of serotonin subtype 1A and negligible dopamine receptor binding [1].
In vivo: BMY 14802 is atypical of standard neuroleptics in that it does not induce catalepsy in rats. In addition, it has been shown to have efficacy in animal models of psychosis [1].
Clinical trial: After 1 week of single-blind placebo treatment, 28 patients were treated with BMY 14802 (up to 3000 mg/day) for up to 4 weeks. However, there was no significant improvement in psychiatric symptoms. There were no changes in involuntary movements, as measured by the Abnormal Involuntary Movement Scale, or in extrapyramidal symptoms as measured by the Simpson-Angus Scale [1].
Reference:
[1] Gewirtz GR, Gorman JM, Volavka J, Macaluso J, Gribkoff G, Taylor DP, Borison R. BMY 14802, a sigma receptor ligand for the treatment of schizophrenia. Neuropsychopharmacology. 1994 Feb;10(1):37-40.
- Ginsenoside Rh3
Catalog No.:BCN1071
CAS No.:105558-26-7
- Fmoc-Glycinol
Catalog No.:BCC3094
CAS No.:105496-31-9
- Calceolarioside B
Catalog No.:BCN2787
CAS No.:105471-98-5
- Risedronate
Catalog No.:BCC4711
CAS No.:105462-24-6
- 7-Epitaxol
Catalog No.:BCN2514
CAS No.:105454-04-4
- Tamoxifen
Catalog No.:BCN1634
CAS No.:10540-29-1
- Spiroxatrine
Catalog No.:BCC6728
CAS No.:1054-88-2
- Bis(3-ethyl-5-methyl-4-maleimidophenyl)methane
Catalog No.:BCC8881
CAS No.:105391-33-1
- Shuterin
Catalog No.:BCN8068
CAS No.:105377-77-3
- Tyrphostin 9
Catalog No.:BCC4471
CAS No.:10537-47-0
- Tanshinlactone
Catalog No.:BCN5867
CAS No.:105351-70-0
- 5,7,4'-Tri-O-methylcatechin
Catalog No.:BCN3951
CAS No.:105330-59-4
- Prostephanaberrine
Catalog No.:BCN4736
CAS No.:105608-27-3
- Fasudil (HA-1077) HCl
Catalog No.:BCC2542
CAS No.:105628-07-7
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- ML 9 hydrochloride
Catalog No.:BCC6644
CAS No.:105637-50-1
- Mizolastine dihydrochloride
Catalog No.:BCC4132
CAS No.:1056596-82-7
- CYT387
Catalog No.:BCC2196
CAS No.:1056634-68-4
- CYT387 sulfate salt
Catalog No.:BCC1506
CAS No.:1056636-06-6
- AT13148
Catalog No.:BCC5360
CAS No.:1056901-62-2
- Androstanolone 17-benzoate
Catalog No.:BCC8825
CAS No.:1057-07-4
- AL 8697
Catalog No.:BCC8037
CAS No.:1057394-06-5
- Ganoderic acid C6
Catalog No.:BCN3257
CAS No.:105742-76-5
- Methyl ganoderate C6
Catalog No.:BCN3259
CAS No.:105742-81-2
A role for sigma binding in the antipsychotic profile of BMY 14802?[Pubmed:8232511]
NIDA Res Monogr. 1993;133:125-57.
BMY 14802 was identified as a potential antipsychotic drug in traditional model systems, and this identification was confirmed in modern behavioral and electrophysiological systems. The drug appears to be atypical as an antipsychotic in its lack of activity in models predictive of the potential to produce extrapyramidal side effects and tardive dyskinesia. Indeed, this suggestion is corroborated by clinical findings to date. The atypical profile of BMY 14802 extends to its neurochemical actions and appears to find its basis in regionally selective, indirect modulation of the dopamine system. Furthermore, BMY 14802 exhibits interactions with sigma binding sites in vitro and in vivo, a notion supported by data from neurophysiological, behavioral, and biochemical investigations. BMY 14802 also appears to be neuroprotective in some model systems and may have utility in the treatment of stroke (Boissard et al. 1991). BMY 14802 appears to interact with 5-HT1A receptors, but this interaction does not seem to contribute significantly to the potential antipsychotic actions of the drug. Moreover, the formation of active metabolites of BMY 14802 does not appear to occur in animals or humans to an extent of physiological or behavioral relevance. If clinically efficacious, BMY 14802 may treat the symptoms of schizophrenia by a mechanism novel for antipsychotic drugs: regionally selective, indirect modulation of dopaminergic systems by specific interaction at sigma sites.
Further characterization of the effects of BMY 14802 on dopamine neuronal activity.[Pubmed:7908761]
Synapse. 1993 Dec;15(4):276-84.
Further evaluation of the effects of BMY 14802 on dopamine (DA) neuronal activity in the rat substantia nigra pars compacta (A9) was conducted with single-unit recording and microiontophoresis in anesthetized rats. Microiontophoretic administration of BMY 14802 (sigma, serotonin (5-HT)-1A and alpha-1 adrenoceptor ligand) had no effect on DA neurons. Microiontophoretic administration of (+)-3-PPP (weak D2 agonist with high affinity for sigma receptors) and quinpirole (D2/D3 agonist) inhibited A9 DA neuronal activity. Co-iontophoresis or i.v. pretreatment with BMY 14802 had no effect on the current-response curves for the effects of microiontophoretic (+)-3-PPP or quinpirole on A9 DA neurons. Co-iontophoretic administration of (-)-sulpiride, a selective D2 antagonist, blocked the inhibitory effects of microiontophoretic (+)-3-PPP. The effects of BMY 14802 (0.25-8 mg/kg, i.v.) on DA neurons (increased firing rate, increased burst-firing, reduced regularity of firing pattern) were not altered by acute brain hemitransection, but were blocked by pretreatment with NAN-190, an antagonist of 5-HT-1A and alpha-1 receptors. The alpha-1 receptor antagonist, prazosin, did not block these effects of BMY 14802. In conclusion, the effects of BMY 14802 on DA neuronal firing rate and firing pattern are indirect, perhaps due in part to the occupation of 5-HT-1A receptors.
Synthesis and biological characterization of alpha-(4-fluorophenyl)-4-(5-fluoro-2-pyrimidinyl)-1-piperazinebutanol and analogues as potential atypical antipsychotic agents.[Pubmed:1361578]
J Med Chem. 1992 Nov 27;35(24):4516-25.
A series of 1-(pyrimidin-2-yl)piperazine derivatives were prepared and evaluated in receptor binding assays and in in vivo behavioral paradigms as potential atypical antipsychotic agents. Compound 16 (BMS 181100 (formerly BMY 14802)) emerged as the lead compound from within the series on the basis of its good activity and duration of action in the inhibition of both conditioned avoidance responding and apomorphine-induced stereotopy in the rat. Compound 16 not only failed to induce catalepsy in the rat but was quite effective in reversing the cataleptic effect of neuroleptic agents, thus indicating a low propensity for causing extrapyramidal side effects. In comparison to reference antipsychotic agents, 16 appeared to be less sedating and was relatively weaker in causing muscle incoordination. The compound was essentially inactive in binding to dopamine D2 receptors and its chronic administration to rats did not result in dopamine receptor supersensitivity. It exhibited modest to weak affinity for 5-HT1A and alpha 1 receptors but was found to be a fairly potent ligand for sigma binding sites (IC50 vs (+)-[3H]-3-PPP = 112 nM). Although the resolved enantiomers of racemic 16 did not show dramatic differences from racemate or from each other in most tests, the R(+) enantiomer was up to 11-fold more potent than its antipode in binding to sigma sites. Several studies have indicated that 16 may be a limbic-selective agent which may modulate dopaminergic activity by an indirect mechanism. The compound has been selected for clinical evaluation in the treatment of psychosis.


