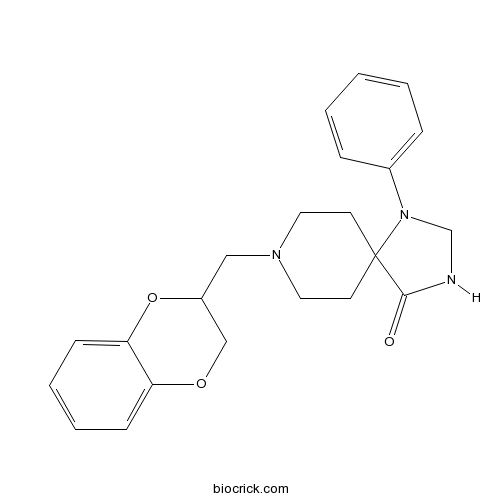
Spiroxatrine5-HT1A antagonist CAS# 1054-88-2 |
- JNK-IN-7
Catalog No.:BCC1672
CAS No.:1408064-71-0
- CC-401 hydrochloride
Catalog No.:BCC1458
CAS No.:1438391-30-0
- CEP 1347
Catalog No.:BCC7982
CAS No.:156177-65-0
- AEG 3482
Catalog No.:BCC8088
CAS No.:63735-71-7
- AS 602801
Catalog No.:BCC1369
CAS No.:848344-36-5
- CC-930
Catalog No.:BCC1459
CAS No.:899805-25-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1054-88-2 | SDF | Download SDF |
| PubChem ID | 5268 | Appearance | Powder |
| Formula | C22H25N3O3 | M.Wt | 379.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in DMSO | ||
| Chemical Name | 8-(2,3-dihydro-1,4-benzodioxin-3-ylmethyl)-1-phenyl-1,3,8-triazaspiro[4.5]decan-4-one | ||
| SMILES | C1CN(CCC12C(=O)NCN2C3=CC=CC=C3)CC4COC5=CC=CC=C5O4 | ||
| Standard InChIKey | JVGBTTIJPBFLTE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H25N3O3/c26-21-22(25(16-23-21)17-6-2-1-3-7-17)10-12-24(13-11-22)14-18-15-27-19-8-4-5-9-20(19)28-18/h1-9,18H,10-16H2,(H,23,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-HT1A antagonist. More active and selective than spiperone. Also a very potent α2C adrenergic receptor antagonist. |

Spiroxatrine Dilution Calculator

Spiroxatrine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6353 mL | 13.1766 mL | 26.3532 mL | 52.7065 mL | 65.8831 mL |
| 5 mM | 0.5271 mL | 2.6353 mL | 5.2706 mL | 10.5413 mL | 13.1766 mL |
| 10 mM | 0.2635 mL | 1.3177 mL | 2.6353 mL | 5.2706 mL | 6.5883 mL |
| 50 mM | 0.0527 mL | 0.2635 mL | 0.5271 mL | 1.0541 mL | 1.3177 mL |
| 100 mM | 0.0264 mL | 0.1318 mL | 0.2635 mL | 0.5271 mL | 0.6588 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bis(3-ethyl-5-methyl-4-maleimidophenyl)methane
Catalog No.:BCC8881
CAS No.:105391-33-1
- Shuterin
Catalog No.:BCN8068
CAS No.:105377-77-3
- Tyrphostin 9
Catalog No.:BCC4471
CAS No.:10537-47-0
- Tanshinlactone
Catalog No.:BCN5867
CAS No.:105351-70-0
- 5,7,4'-Tri-O-methylcatechin
Catalog No.:BCN3951
CAS No.:105330-59-4
- Neocaesalpin O
Catalog No.:BCN7266
CAS No.:1053189-53-9
- Aloeresin D
Catalog No.:BCN2850
CAS No.:105317-67-7
- Ganodermatriol
Catalog No.:BCC8177
CAS No.:105300-28-5
- 3'-Deoxy-4-O-methylepisappanol
Catalog No.:BCN3676
CAS No.:1052714-12-1
- 5'-Methoxylariciresinol
Catalog No.:BCN7012
CAS No.:105256-12-0
- SBE 13 HCl
Catalog No.:BCC6408
CAS No.:1052532-15-6
- Virosine B
Catalog No.:BCN6742
CAS No.:1052228-70-2
- Tamoxifen
Catalog No.:BCN1634
CAS No.:10540-29-1
- 7-Epitaxol
Catalog No.:BCN2514
CAS No.:105454-04-4
- Risedronate
Catalog No.:BCC4711
CAS No.:105462-24-6
- Calceolarioside B
Catalog No.:BCN2787
CAS No.:105471-98-5
- Fmoc-Glycinol
Catalog No.:BCC3094
CAS No.:105496-31-9
- Ginsenoside Rh3
Catalog No.:BCN1071
CAS No.:105558-26-7
- BMY 14802 hydrochloride
Catalog No.:BCC5759
CAS No.:105565-55-7
- Prostephanaberrine
Catalog No.:BCN4736
CAS No.:105608-27-3
- Fasudil (HA-1077) HCl
Catalog No.:BCC2542
CAS No.:105628-07-7
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- ML 9 hydrochloride
Catalog No.:BCC6644
CAS No.:105637-50-1
- Mizolastine dihydrochloride
Catalog No.:BCC4132
CAS No.:1056596-82-7
Involvement of the first transmembrane segment of human alpha(2) -adrenoceptors in the subtype-selective binding of chlorpromazine, spiperone and spiroxatrine.[Pubmed:21649638]
Br J Pharmacol. 2011 Nov;164(5):1558-72.
BACKGROUND AND PURPOSE: Some large antagonist ligands (ARC239, chlorpromazine, prazosin, spiperone, Spiroxatrine) bind to the human alpha(2A) -adrenoceptor with 10- to 100-fold lower affinity than to the alpha(2B)- and alpha(2C)-adrenoceptor subtypes. Previous mutagenesis studies have not explained this subtype selectivity. EXPERIMENTAL APPROACH: The possible involvement of the extracellular amino terminus and transmembrane domain 1 (TM1) in subtype selectivity was elucidated with eight chimaeric receptors: six where TM1 and the N-terminus were exchanged between the alpha(2)-adrenoceptor subtypes and two where only TM1 was exchanged. Receptors were expressed in CHO cells and tested for ligand binding with nine chemically diverse antagonist ligands. For purposes of interpretation, molecular models of the three human alpha(2)-adrenoceptors were constructed based on the beta(2)-adrenoceptor crystal structure. KEY RESULTS: The affinities of three antagonists (spiperone, Spiroxatrine and chlorpromazine) were significantly improved by TM1 substitutions of the alpha(2A)-adrenoceptor, but reciprocal effects were not seen for chimaeric receptors based on alpha(2B)- and alpha(2C)-adrenoceptors. Molecular docking of these ligands suggested that binding occurs in the orthosteric ligand binding pocket. CONCLUSIONS AND IMPLICATIONS: TM1 is involved in determining the low affinity of some antagonist ligands at the human alpha(2A)-adrenoceptor. The exact mechanism is not known, but the position of TM1 at a large distance from the binding pocket indicates that TM1 does not participate in specific side-chain interactions with amino acids within the binding pocket of the receptor or with ligands bound therein. Instead, molecular models suggest that TM1 has indirect conformational effects related to the charge distribution or overall shape of the binding pocket.
[The alpha-antiadrenergic properties of spiroxatrine, a ligand of serotonergic 5-HT1A receptors].[Pubmed:8105762]
Arch Inst Cardiol Mex. 1993 Jul-Aug;63(4):289-95.
The 5-HT1A ligand, Spiroxatrine, displays very low affinity for alpha 1-adrenergic binding sites and a relatively high affinity for alpha 2-adrenergic binding sites. Nonetheless, recent functional studies indicate that Spiroxatrine is a potent antagonist of the alpha 1-adrenoceptor mediating contraction in the rat isolated aorta. On the basis of the widely studied heterogeneous interaction of drugs with alpha-adrenoceptors in several experimental models, the present study was designed to analyze the alpha-adrenoceptor antagonist properties of Spiroxatrine in the pithed rat. Animals were prepared for recording of arterial blood pressure and intravenous (i.v.) administration of drugs. Norepinephrine and the alpha 1- and alpha 2- adrenoceptor agonists methoxamine and clonidine, respectively, elicited pressor responses in a dose-related fashion. Spiroxatrine (1 mg/kg, i.v.) produced a moderate--but significant--rightward displacement of the dose-response curves to all agonists. The present data lead us to suggest that, though Spiroxatrine exhibits alpha 1- and alpha 2-adrenoceptor antagonist properties in the pithed rat, its potency does not seem to correlate with that found in rat aorta. The potential involvement of alpha 1-adrenoceptor subtypes is discussed.
Alpha 1-adrenoceptor blocking properties of spiroxatrine in rat aorta.[Pubmed:1352025]
Life Sci. 1992;51(1):PL1-6.
This preliminary study has analyzed the potential ability of the 5-HT1A ligand Spiroxatrine to interact with vascular alpha 1-adrenoceptors. Norepinephrine and the selective alpha 1-adrenoceptor agonist, methoxamine, elicited concentration-dependent contractions of rat aortic rings. In contrast, (+/-)-Spiroxatrine (from 10(-8) to 3.1X10(-7) M) was devoid of any effect on vascular tone per se, but shifted the concentration-response curves of norepinephrine and methoxamine to the right in a concentration-dependent manner with pA2 values of 8.48 +/- 0.22 and 8.93 +/- 0.33, respectively. Endothelium removal did not significantly affect the above pA2 values of (+/-)-Spiroxatrine. These data, taken in concert, support the contention that (+/-)-Spiroxatrine displays alpha 1-adrenoceptor blocking properties in rat aortic rings.
Spiroxatrine augments fluoxetine-induced reduction of ethanol intake by the P line of rats.[Pubmed:2533688]
Pharmacol Biochem Behav. 1989 Oct;34(2):381-6.
The present study was undertaken to determine if Spiroxatrine, a reported 5-HT1A antagonist, could block the attenuating effects of fluoxetine (a 5-HT uptake inhibitor) on voluntary ethanol intake by the selectively bred alcohol-preferring P line of rats. Fluoxetine (10 mg/kg, IP) significantly reduced the intake of 10% ethanol by P rats approximately 50% during the 4-hour period of alcohol availability. Spiroxatrine (4 mg/kg, IP) was without effect on ethanol intake when given alone. However, when given 5 minutes before fluoxetine (10 mg/kg, IP), this dose of Spiroxatrine augmented the reduction of ethanol intake to approximately 15% of control values after 4 hours. Similar experiments conducted with 1 mg/kg (IP) 8-hydroxy-2(di-N-propylamino) tetralin (DPAT) demonstrated that this 5-HT1A agonist also enhanced the attenuating effects of fluoxetine on ethanol intake. Likewise, Spiroxatrine augmented the DPAT reduction of alcohol intake. Spiroxatrine enhanced the effect of DPAT and fluoxetine on food intake as it did on ethanol intake. The results suggest that Spiroxatrine behaved as a partial agonist and/or modulator and not as an antagonist at 5-HT1A receptors under the present experimental conditions.
Pharmacological characteristics of alpha 2-adrenergic receptors: comparison of pharmacologically defined subtypes with subtypes identified by molecular cloning.[Pubmed:1353247]
Mol Pharmacol. 1992 Jul;42(1):1-5.
On the basis of extensive radioligand data and more limited functional data, three pharmacological subtypes of alpha 2-adrenergic receptors have been identified. More recently, three human genes or cDNAs for alpha 2-adrenergic receptors have been identified by molecular cloning. The relationship, however, among the pharmacologically defined subtypes and those identified by molecular cloning has not been clear. In order to resolve this issue, we have compared the pharmacological characteristics of the receptors identified by molecular cloning and expressed in COS-7 cells with the characteristics of the pharmacologically defined receptors in their respective prototypic tissue or cell line. The affinities (Ki values) of 12 subtype-selective alpha 2-adrenergic antagonists were determined for the alpha 2 receptor in the six preparations, by radioligand binding. Correlation analyses of the pKi values indicate that the alpha 2A subtype, as defined in the HT29 cell line, the alpha 2B receptor of the neonatal rat lung, and the alpha 2C subtype, as defined in an oppossum kidney cell line, correspond to the cloned human alpha 2-C10, alpha 2-C2, and alpha 2-C4 receptor subtypes, respectively.
Centrally acting hypotensive agents with affinity for 5-HT1A binding sites inhibit forskolin-stimulated adenylate cyclase activity in calf hippocampus.[Pubmed:3207999]
Br J Pharmacol. 1988 Nov;95(3):975-85.
1. A number of centrally acting hypotensive agents and other ligands with high affinity for 5-hydroxytryptamine1A (5-HT1A) recognition sites have been tested on forskolin-stimulated adenylate cyclase activity in calf hippocampus, a functional model for 5-HT1A-receptors. 2. Concentration-dependent inhibition of forskolin-stimulated adenylate cyclase activity was elicited by the reference 5-HT1-receptor agonists (mean EC50 value, nM): 5-HT (22), 5-carboxamidotryptamine (5-CT, 3.2), 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT, 8.6), N,N-dipropyl-5-carboxamidotryptamine (DP-5-CT, 2.3), 1-[2-(4-aminophenyl)ethyl]-4-(3-trifluoromethylphenyl)-piperazine (PAPP or LY 165163, 20), 5-methoxy-3-(1,2,3,6-tetrahydro-4-pyridinyl)-1H indole (RU 24969, 20), buspirone (65) and ipsapirone (56). Emax amounted to 18-20% inhibition for all but the latter two agonists (14%). 3. The following hypotensive agents with high affinity for 5-HT1A sites were potent agonists in this system (mean EC50 value, nM): flesinoxan (24), indorenate (99), erythro-1-(1-[2-(1,4-benzodioxan-2-yl)-2-hydroxyethyl]-4-piperidyl )- 2-benzimidazolinone (R 28935, 2.5), urapidil (390) and 5-methyl-urapidil (3.5). The first two agents were full agonists, whereas the latter three acted as partial agonists with 60-80% efficacy. 4. Metergoline and methysergide behaved as full agonists and cyanopindolol as a partial agonist with low efficacy. Spiroxatrine and 2-(2,6-dimethoxyphenoxyethyl)aminomethyl- 1,4-benzodioxane (WB 4101) which bind to 5-HT1A sites with nanomolar affinity, were agonists and inhibited potently forskolin-stimulated adenylate cyclase in calf hippocampus, showing mean EC50 values of 23 and 15 nM, respectively. Spiroxatrine and WB 4101 yielded 90% and 50% efficacy, respectively. 5. Spiperone and methiothepin (each 1 microM) caused rightward shifts of the concentration-effect curve to 8-OH-DPAT, without loss of the maximal effect, as did the partial agonist cyanopindolol (0.1 microM) and the (-)- and (+)-enantiomers of pindolol (1 microM and 0.1 mM, respectively). 6. There was an excellent correlation (r = 0.90, P = 0.0001) between the pEC50 values (ranging from 6.4 to 8.7) of the 19 agonists tested at adenylate cyclase and their pKD for 5-HT1A recognition sites. Apparent pKB values of antagonists at adenylate cyclase and their pKD values for 5-HT1A binding sites were also significantly correlated. 7. This study further indicates that the 5-HT1A recognition site and the 5-HT receptor mediating inhibition of adenylate cyclase in hippocampus are the same. The data show that a number of centrally acting hypotensive agents with high affinity for the 5-HT1,A site are potent agonists in this model, suggesting an involvement of central 5-HTIA-receptors in the control of blood pressure.


