A 804598P2X7 antagonist,potent and selective CAS# 1125758-85-1 |
- KX2-391 dihydrochloride
Catalog No.:BCC1686
CAS No.:1038395-65-1
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- Dasatinib hydrochloride
Catalog No.:BCC1517
CAS No.:854001-07-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1125758-85-1 | SDF | Download SDF |
| PubChem ID | 53325874 | Appearance | Powder |
| Formula | C19H17N5 | M.Wt | 315.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 32 mg/mL (101.47 mM) *"≥" means soluble, but saturation unknown. | ||
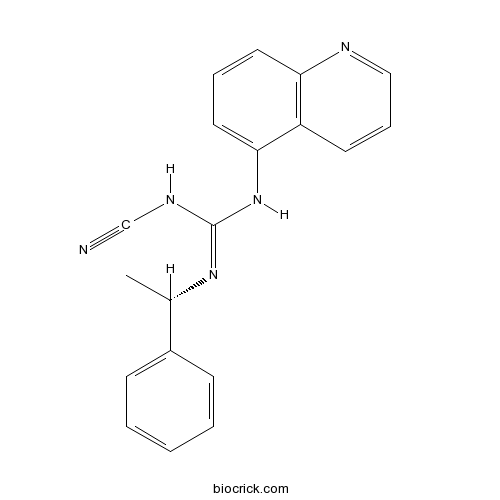
| Chemical Name | 1-cyano-2-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine | ||
| SMILES | CC(C1=CC=CC=C1)N=C(NC#N)NC2=CC=CC3=C2C=CC=N3 | ||
| Standard InChIKey | PQYCRDPLPKGSME-AWEZNQCLSA-N | ||
| Standard InChI | InChI=1S/C19H17N5/c1-14(15-7-3-2-4-8-15)23-19(22-13-20)24-18-11-5-10-17-16(18)9-6-12-21-17/h2-12,14H,1H3,(H2,22,23,24)/t14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, competitive P2X7 receptor antagonist (IC50 values are 8.9, 9.9 and 10.9 nM for mouse, rat and human P2X7 receptors respectively). Selective for P2X7 receptors (IC50 > 5-10 μM for a wide array of cell surface receptors and ion channels). Binds with high affinity (Ki app = 2.4 nM for rat P2X7 receptors). |

A 804598 Dilution Calculator

A 804598 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1709 mL | 15.8544 mL | 31.7088 mL | 63.4176 mL | 79.272 mL |
| 5 mM | 0.6342 mL | 3.1709 mL | 6.3418 mL | 12.6835 mL | 15.8544 mL |
| 10 mM | 0.3171 mL | 1.5854 mL | 3.1709 mL | 6.3418 mL | 7.9272 mL |
| 50 mM | 0.0634 mL | 0.3171 mL | 0.6342 mL | 1.2684 mL | 1.5854 mL |
| 100 mM | 0.0317 mL | 0.1585 mL | 0.3171 mL | 0.6342 mL | 0.7927 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Mps1-IN-1
Catalog No.:BCC5590
CAS No.:1125593-20-5
- 4-O-Methylepisappanol
Catalog No.:BCN3674
CAS No.:112529-37-0
- Pioglitazone HCl
Catalog No.:BCC2278
CAS No.:112529-15-4
- ent-16alpha,17-Dihydroxyatisan-3-one
Catalog No.:BCN6607
CAS No.:112523-91-8
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
- Citrusinol
Catalog No.:BCN8083
CAS No.:112516-43-5
- Tubuloside A
Catalog No.:BCN2806
CAS No.:112516-05-9
- Neolinine
Catalog No.:BCN6564
CAS No.:112515-37-4
- Isoabsouline
Catalog No.:BCN1955
CAS No.:112513-34-5
- Absouline
Catalog No.:BCN1954
CAS No.:112513-33-4
- Picrasidine S
Catalog No.:BCN6006
CAS No.:112503-87-4
- Aristolactam FI
Catalog No.:BCN6005
CAS No.:112501-42-5
- SKLB610
Catalog No.:BCC3647
CAS No.:1125780-41-7
- 4-Allylpyrocatechol
Catalog No.:BCN6009
CAS No.:1126-61-0
- Iso-mogroside V
Catalog No.:BCN3047
CAS No.:1126032-65-2
- Dicyclanil
Catalog No.:BCC8938
CAS No.:112636-83-6
- U-73122
Catalog No.:BCC5199
CAS No.:112648-68-7
- Garcinone E
Catalog No.:BCN3604
CAS No.:112649-21-5
- BR-Xanthone A
Catalog No.:BCN6007
CAS No.:112649-48-6
- 1,7-Dihydroxyacridone
Catalog No.:BCN7275
CAS No.:112649-95-3
- Fragransin A2
Catalog No.:BCN6008
CAS No.:112652-46-7
- Erigeside C
Catalog No.:BCN6010
CAS No.:112667-09-1
- Oleonuezhenide
Catalog No.:BCN6011
CAS No.:112693-21-7
- IWR-1-endo
Catalog No.:BCC5102
CAS No.:1127442-82-3
The impact of the P2X7 receptor antagonist A-804598 on neuroimmune and behavioral consequences of stress.[Pubmed:25083574]
Behav Pharmacol. 2014 Sep;25(5-6):582-98.
Stress leads to neuroinflammatory and behavioral consequences through upregulation of inflammatory-related cytokines within the central nervous system such as interleukin-1beta (IL-1beta), which may be indicative of microglial priming/activation. Evidence suggests that the P2X7 receptor (P2X7R) may play an important role in the synthesis and conversion of IL-1beta. In a series of six experiments, adult male rats were intubated with a highly selective P2X7R antagonist (A-804598) before footshock exposure. As expected, footshock increased IL-1beta and CD14 mRNA in the paraventricular nucleus, and A-804598 (25 mg/kg) partially attenuated these effects. Footshock also increased hypothalamic IL-1 protein in whole hypothalamic blocks, but no effect was observed on the formation of pro-IL-1beta or IL-1beta in the paraventricular nucleus as assessed using western blotting. A-804598 also did not reverse the suppression in exploration produced by stress exposure. The present findings support the use of the footshock paradigm as a method for inducing stress-related neuroimmune and behavioral changes, but the evidence to support the role of A-804598 as a potential tool to reverse such changes remains modest. This study is the first to examine the role of P2X7R in vivo following footshock exposure. Further characterization of P2X7R may have implications for understanding the relationship between stress and inflammation.
[3H]A-804598 ([3H]2-cyano-1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine) is a novel, potent, and selective antagonist radioligand for P2X7 receptors.[Pubmed:18602931]
Neuropharmacology. 2009 Jan;56(1):223-9.
ATP-sensitive P2X7 receptors are localized on cells of immunological origin including peripheral macrophages and glial cells in the CNS. Activation of P2X7 receptors leads to rapid changes in intracellular calcium concentrations, release of the pro-inflammatory cytokine IL-1beta, and following prolonged agonist exposure, the formation of cytolytic pores in plasma membranes. Data from gene knockout studies and recently described selective antagonists indicate a role for P2X7 receptor activation in inflammation and pain. While several species selective P2X7 antagonists exist, A-804598 represents a structurally novel, competitive, and selective antagonist that has equivalent high affinity at rat (IC50 = 10 nM), mouse (IC50 = 9 nM) and human (IC50 = 11 nM) P2X7 receptors. A-804598 also potently blocked agonist stimulated release of IL-1beta and Yo-Pro uptake from differentiated THP-1 cells that natively express human P2X7 receptors. A-804598 was tritiated ([3H]A-804598; 8.1Ci/mmol) and utilized to study recombinant rat P2X7 receptors expressed in 1321N1 cells. [3H]A-804598 labeled a single class of high affinity binding sites (Kd=2.4 nM and apparent Bmax=0.56 pmol/mg). No specific binding was observed in untransfected 1321N1 cells. The pharmacological profile for P2X antagonists to inhibit [3H]A-804598 binding correlated with their ability to block functional activation of P2X7 receptors (r=0.95, P<0.05). These data demonstrate that A-804598 is one of the most potent and selective antagonists for mammalian P2X7 receptors described to date and [3H]A-804598 is a high affinity antagonist radioligand that specifically labels rat P2X7 receptors.
Receptor localization, native tissue binding and ex vivo occupancy for centrally penetrant P2X7 antagonists in the rat.[Pubmed:20840537]
Br J Pharmacol. 2011 Jan;162(2):405-14.
BACKGROUND AND PURPOSE: The P2X7 receptor is implicated in inflammation and pain and is therefore a potential target for therapeutic intervention. Here, the development of a native tissue radioligand binding, localization and ex vivo occupancy assay for centrally penetrant P2X7 receptor antagonists is described. EXPERIMENTAL APPROACH: Autoradiography studies using the P2X7 antagonist radioligand [(3)H]-A-804598 were carried out in rat brain and spinal cord. Subsequent in vitro binding and ex vivo occupancy assays were performed using rat cortex homogenate. KEY RESULTS: P2X7 expression was shown to be widespread throughout the rat brain, and in the grey matter of the spinal cord. In binding assays in rat cortex homogenate, approximately 60% specific binding was achieved at equilibrium. In kinetic binding assays, k(on) and k(off) values of 0.0021.min(-)(1).nM(-)(1) and 0.0070.min(-)(1) were determined, and the K(d) derived from kinetic measurements was consistent with that derived from saturation analysis. Novel P2X7 antagonists inhibited the binding of [(3)H]-A-804598 to rat cortex P2X7 receptors with K(i) values of <40 nM. In an ex vivo occupancy assay, a P2X7 antagonist dosed orally to rats caused a concentration-dependent inhibition of the specific binding of [(3)H]-A-804598 to rat cortex. CONCLUSIONS AND IMPLICATIONS: The present study describes the development of an assay that allows localization of P2X7 receptors, the measurement of the binding affinity of P2X7 receptor antagonists in native tissue, and provides a means of determining central P2X7 receptor occupancy. These assays could form an important part of a P2X7 drug discovery programme.


