1,7-DihydroxyacridoneCAS# 112649-95-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 112649-95-3 | SDF | Download SDF |
| PubChem ID | 13857937 | Appearance | Powder |
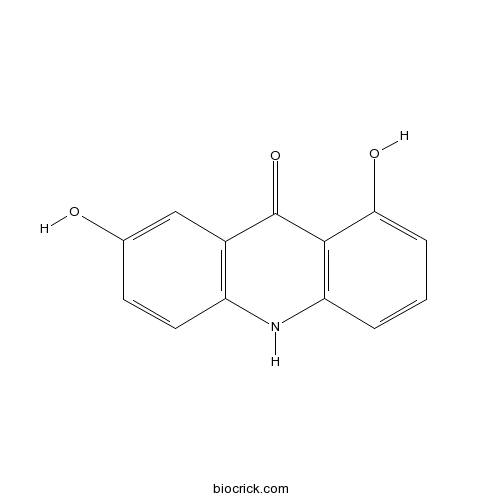
| Formula | C13H9NO3 | M.Wt | 227.21 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,7-dihydroxy-10H-acridin-9-one | ||
| SMILES | C1=CC2=C(C(=C1)O)C(=O)C3=C(N2)C=CC(=C3)O | ||
| Standard InChIKey | KXKWBGSBYIZPDQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H9NO3/c15-7-4-5-9-8(6-7)13(17)12-10(14-9)2-1-3-11(12)16/h1-6,15-16H,(H,14,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1,7-Dihydroxyacridone is a natural product from Boenninghausenia albiflora. |
| Structure Identification | Supplements to Edition of Rodds Chemistry of Carbon Compounds, 1975:245-258.Sainsbury M. Chapter 32–The acridine alkaloids.[Reference: WebLink]This chapter provides an overview of the acridine alkaloids. Gravacridone triol represents a new alkaloid from Ruta graveolens, where it co-occurs with its monoglucosyl derivative and the glucoside of a known alkaloid gravacridone diol.
|

1,7-Dihydroxyacridone Dilution Calculator

1,7-Dihydroxyacridone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4012 mL | 22.0061 mL | 44.0121 mL | 88.0243 mL | 110.0304 mL |
| 5 mM | 0.8802 mL | 4.4012 mL | 8.8024 mL | 17.6049 mL | 22.0061 mL |
| 10 mM | 0.4401 mL | 2.2006 mL | 4.4012 mL | 8.8024 mL | 11.003 mL |
| 50 mM | 0.088 mL | 0.4401 mL | 0.8802 mL | 1.7605 mL | 2.2006 mL |
| 100 mM | 0.044 mL | 0.2201 mL | 0.4401 mL | 0.8802 mL | 1.1003 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- BR-Xanthone A
Catalog No.:BCN6007
CAS No.:112649-48-6
- Garcinone E
Catalog No.:BCN3604
CAS No.:112649-21-5
- U-73122
Catalog No.:BCC5199
CAS No.:112648-68-7
- Dicyclanil
Catalog No.:BCC8938
CAS No.:112636-83-6
- Iso-mogroside V
Catalog No.:BCN3047
CAS No.:1126032-65-2
- 4-Allylpyrocatechol
Catalog No.:BCN6009
CAS No.:1126-61-0
- SKLB610
Catalog No.:BCC3647
CAS No.:1125780-41-7
- A 804598
Catalog No.:BCC6198
CAS No.:1125758-85-1
- Mps1-IN-1
Catalog No.:BCC5590
CAS No.:1125593-20-5
- 4-O-Methylepisappanol
Catalog No.:BCN3674
CAS No.:112529-37-0
- Pioglitazone HCl
Catalog No.:BCC2278
CAS No.:112529-15-4
- ent-16alpha,17-Dihydroxyatisan-3-one
Catalog No.:BCN6607
CAS No.:112523-91-8
- Fragransin A2
Catalog No.:BCN6008
CAS No.:112652-46-7
- Erigeside C
Catalog No.:BCN6010
CAS No.:112667-09-1
- Oleonuezhenide
Catalog No.:BCN6011
CAS No.:112693-21-7
- IWR-1-endo
Catalog No.:BCC5102
CAS No.:1127442-82-3
- exo-IWR 1
Catalog No.:BCC7823
CAS No.:1127442-87-8
- Galanin (1-15) (porcine, rat)
Catalog No.:BCC5762
CAS No.:112747-70-3
- Clemastanin B
Catalog No.:BCC8152
CAS No.:112747-98-5
- Osthenone
Catalog No.:BCN4731
CAS No.:112789-90-9
- Letrozole
Catalog No.:BCC1063
CAS No.:112809-51-5
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- 1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid ethyl ester
Catalog No.:BCC8465
CAS No.:112811-71-9
- 1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid
Catalog No.:BCC8464
CAS No.:112811-72-0
Sainsbury M. Chapter 32–The acridine alkaloids.
Supplements to Edition of Rodds Chemistry of Carbon Compounds, 1975:245-258.
This chapter provides an overview of the acridine alkaloids. Gravacridone triol represents a new alkaloid from Ruta graveolens, where it co-occurs with its monoglucosyl derivative and the glucoside of a known alkaloid gravacridone diol. The 5-hydroxy derivative of the known alkaloid arborinine has been obtained for the first time from the leaves of Glycosmis bilocularis. Two other acridones differing only in the presence or absence of methoxyl groups are the alkaloids and isolated from the leaves of Bauerella simplicifolia. Boenninghausenia albiflora produces 1-hydroxyacridone and possibly also 1,7-Dihydroxyacridone. Two new acridones, 1-hydroxy-3,4-dimethoxy-10-methylacridone and l-hydroxy-3-geranyloxy-4-methoxy-10-methylacridone occur in extracts of the plant Sarcomelicope leiocarpa indigenous to New Caledonia together with eight other known structures. Another series of related alkaloids are produced by the plant Glycosmis citrifolia; these include the acridones glycocitrine-I, glycocitrine-II, its o-methyl ether, glyfoline, furofoline-II, and pyranofoline.


