DehydroeffusolCAS# 137319-34-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 137319-34-7 | SDF | Download SDF |
| PubChem ID | 5316810 | Appearance | Powder |
| Formula | C17H14O2 | M.Wt | 250.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
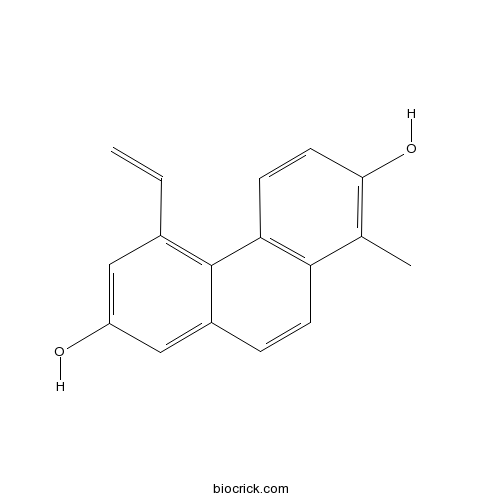
| Chemical Name | 5-ethenyl-1-methylphenanthrene-2,7-diol | ||
| SMILES | CC1=C(C=CC2=C1C=CC3=CC(=CC(=C32)C=C)O)O | ||
| Standard InChIKey | GSSPKCPIRDPBQE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14O2/c1-3-11-8-13(18)9-12-4-5-14-10(2)16(19)7-6-15(14)17(11)12/h3-9,18-19H,1H2,2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Dehydroeffusol inhibits gastric cancer cell growth and tumorigenicity by selectively inducing tumor-suppressive endoplasmic reticulum stress and a moderate apoptosis. 2. Dehydroeffusol effectively inhibits human gastric cancer cell-mediated vasculogenic mimicry with low toxicity, it is a potential drug candidate for anti-gastric cancer neovascularization and anti-gastric cancer therapy. 3. Dehydroeffusol may antagonize the spasmogenic activity of various agents, and therefore, could be a promising agent in the treatment of spasms. 4. Dehydroeffusol possesses anxiolytic and sedative properties and does not affect the general movement coordination of mice. 5. Dehydroeffusol displays enhanced antimicrobial activities in light, its antimicrobial activities (minimum inhibitory concentrations) against methicillin-resistant and -sensitive Staphylococcus aureus and Candida albicans are increased 16 fold by irradiation with ultraviolet A (UVA). |
| Targets | MMP(e.g.TIMP) | Antifection |

Dehydroeffusol Dilution Calculator

Dehydroeffusol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9952 mL | 19.976 mL | 39.9521 mL | 79.9041 mL | 99.8801 mL |
| 5 mM | 0.799 mL | 3.9952 mL | 7.9904 mL | 15.9808 mL | 19.976 mL |
| 10 mM | 0.3995 mL | 1.9976 mL | 3.9952 mL | 7.9904 mL | 9.988 mL |
| 50 mM | 0.0799 mL | 0.3995 mL | 0.799 mL | 1.5981 mL | 1.9976 mL |
| 100 mM | 0.04 mL | 0.1998 mL | 0.3995 mL | 0.799 mL | 0.9988 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4,5-Dioxo-4,5-seco-11(13)-cadinen-12-oic acid
Catalog No.:BCN1577
CAS No.:137288-61-0
- 4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pymol[2,3-d]pyrimodin-5-yl)ethyl]benzoic acid
Catalog No.:BCC8670
CAS No.:137281-39-1
- Pemetrexed
Catalog No.:BCC9115
CAS No.:137281-23-3
- Boc-Gln-OH
Catalog No.:BCC3382
CAS No.:13726-85-7
- Boc-Glu(OtBu)-OH
Catalog No.:BCC3392
CAS No.:13726-84-6
- Boc-Hyp-OH
Catalog No.:BCC3251
CAS No.:13726-69-7
- Boc-Asp-OH
Catalog No.:BCC2609
CAS No.:13726-67-5
- Chlorantholide E
Catalog No.:BCN4836
CAS No.:1372558-36-5
- Chlorantholide C
Catalog No.:BCN4837
CAS No.:1372558-35-4
- Chlorantholide B
Catalog No.:BCN4834
CAS No.:1372558-34-3
- Chlorantholide A
Catalog No.:BCN4835
CAS No.:1372558-33-2
- GSK2636771
Catalog No.:BCC4993
CAS No.:1372540-25-4
- Cathepsin S inhibitor
Catalog No.:BCC1455
CAS No.:1373215-15-6
- LY 215840
Catalog No.:BCC7101
CAS No.:137328-52-0
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- Boc-Lys-OH
Catalog No.:BCC3410
CAS No.:13734-28-6
- Boc-Phe-OH
Catalog No.:BCC3432
CAS No.:13734-34-4
- Boc-Sar-OH
Catalog No.:BCC3337
CAS No.:13734-36-6
- Boc-Ser(tBu)-OH
Catalog No.:BCC3444
CAS No.:13734-38-8
- Boc-Thr(tBu)-OH
Catalog No.:BCC3452
CAS No.:13734-40-2
- Boc-Val-OH
Catalog No.:BCC3465
CAS No.:13734-41-3
- GSK J1
Catalog No.:BCC2231
CAS No.:1373422-53-7
- GSK J4 HCl
Catalog No.:BCC2230
CAS No.:1373423-53-0
- Nω-Propyl-L-arginine hydrochloride
Catalog No.:BCC6965
CAS No.:137361-05-8
Dehydroeffusol effectively inhibits human gastric cancer cell-mediated vasculogenic mimicry with low toxicity.[Pubmed:25982451]
Toxicol Appl Pharmacol. 2015 Sep 1;287(2):98-110.
Accumulated data has shown that various vasculogenic tumor cells, including gastric cancer cells, are able to directly form tumor blood vessels via vasculogenic mimicry, supplying oxygen and nutrients to tumors, and facilitating progression and metastasis of malignant tumors. Therefore, tumor vasculogenic mimicry is a rational target for developing novel anticancer therapeutics. However, effective antitumor vasculogenic mimicry-targeting drugs are not clinically available. In this study, we purified 2,7-dihydroxyl-1-methyl-5-vinyl-phenanthrene, termed Dehydroeffusol, from the traditional Chinese medicinal herb Juncus effusus L., and found that Dehydroeffusol effectively inhibited gastric cancer cell-mediated vasculogenic mimicry in vitro and in vivo with very low toxicity. Dehydroeffusol significantly suppressed gastric cancer cell adhesion, migration, and invasion. Molecular mechanistic studies revealed that Dehydroeffusol markedly inhibited the expression of a vasculogenic mimicry master gene VE-cadherin and reduced adherent protein exposure on the cell surface by inhibiting gene promoter activity. In addition, Dehydroeffusol significantly decreased the expression of a key vasculogenic gene matrix metalloproteinase 2 (MMP2) in gastric cancer cells, and diminished MMP2 protease activity. Together, our results showed that Dehydroeffusol effectively inhibited gastric cancer cell-mediated vasculogenic mimicry with very low toxicity, suggesting that Dehydroeffusol is a potential drug candidate for anti-gastric cancer neovascularization and anti-gastric cancer therapy.
Effects of dehydroeffusol on spasmogen-induced contractile responses of rat intestinal smooth muscles.[Pubmed:25089735]
Planta Med. 2014 Aug;80(12):978-83.
Dehydroeffusol is a naturally occurring phenanthrene isolated from Juncus effusus. In the context of screening new drugs against gastrointestinal spasms, we investigated its effects on isolated rat jejunum in vitro. Dehydroeffusol (30-90 microM) slightly and transiently enhanced contractions in a concentration-dependent manner but significantly inhibited the contractions induced by KCl (100 mM), (+/-)-Bay-K8644 (5 microM), pilocarpine (90 microM), and histamine (100 microM). These results show that Dehydroeffusol may antagonize the spasmogenic activity of various agents, and therefore, could be a promising agent in the treatment of spasms. Its potential spasmolytic mechanism is also discussed.
Dehydroeffusol inhibits gastric cancer cell growth and tumorigenicity by selectively inducing tumor-suppressive endoplasmic reticulum stress and a moderate apoptosis.[Pubmed:26774454]
Biochem Pharmacol. 2016 Mar 15;104:8-18.
Gastric cancer is ranked as the third leading cause of cancer-related death in the world. Although extensive efforts have been made in recent decades to treat gastric cancer with various anticancer drugs, effective anti-gastric cancer therapeutics to cure the disease are still lacking in the clinics. Therefore, potent novel anti-gastric cancer drugs are greatly needed. In this study, we explored a novel anti-gastric cancer agent from a medicinal herb named Juncus effusus and found that the active component Dehydroeffusol (DHE), a small molecular phenanthrene, effectively inhibited gastric cancer cell proliferation and tumorigenesis by inducing tumor suppressive endoplasmic reticulum (ER) stress and by triggering moderate apoptosis. Mechanistic studies revealed that DHE selectively activated the intracellular tumor suppressive stress response by promoting the overexpression of the key ER stress marker DNA damage-inducible transcript 3 (DDIT3), through upregulation of activating transcription factor 4 (ATF4). Concurrently, DHE suppressed the expression of the cell survival and ER stress marker glucose regulated protein of molecular mass 78 (GRP78) via downregulation of the transcription factor ATF6. In addition, DHE markedly activated the stress response signaling pathway MEKK4-MKK3/6-p38-DDIT3, but significantly inhibited ERK signaling. Our data suggest that DHE inhibits gastric cancer cell growth and tumorigenicity through selectively inducing a robust tumor suppressive ER stress response and a moderate apoptosis response. Therefore, DHE may provide a novel drug candidate for further development of potential anti-gastric cancer therapeutics.
Anxiolytic and sedative effects of dehydroeffusol from Juncus effusus in mice.[Pubmed:21104609]
Planta Med. 2011 Mar;77(5):416-20.
Dehydroeffusol, a phenanthrene isolated from Juncus effusus L., possesses characteristic anxiolytic and sedative properties, as determined by an array of behavioral tests in mice. In the elevated plus-maze test, Dehydroeffusol significantly increased the number of entries into the open arms and the time the mice spent in these arms in a dose-dependent manner, with a minimum effective dose of 2.5 mg/kg. Dehydroeffusol also significantly increased the head-dips of mice in the hole-board test in a dose-dependent manner, with a minimum effective dose of 5 mg/kg. Dehydroeffusol reduced mouse locomotion in the open-field test with a minimum effective dose of 5 mg/kg. In the rota-rod test, 1-5 mg/kg Dehydroeffusol did not decrease the fall-down time of mice. The above results confirm that Dehydroeffusol possesses anxiolytic and sedative properties and does not affect the general movement coordination of mice. This suggests that Dehydroeffusol is a novel anxiolytic chemical derived from herbal medicines.
Antimicrobial DNA-binding photosensitizers from the common rush, Juncus effusus.[Pubmed:12126307]
Photochem Photobiol. 2002 Jul;76(1):51-6.
Our continuing survey of phototoxins from higher plants has led to the isolation and identification from the common rush, Juncus effusus L., of the phenanthrene, Dehydroeffusol (1), and the dihydrophenanthrene, juncusol (2), compounds that display enhanced antimicrobial activities in light. The antimicrobial activities (minimum inhibitory concentrations) for these compounds against methicillin-resistant and -sensitive Staphylococcus aureus and Candida albicans were increased 16- and two-fold, respectively, by irradiation with ultraviolet A (UVA). Photosensitized DNA-binding activities (as possible covalent bond formation) of these compounds were determined by using restriction enzymes and a specially prepared 1.5 kb DNA fragment. Under UVA irradiation, Dehydroeffusol strongly inhibited all the restriction enzymes (KpnI, XbaI, PmeI, DraI, PacI and BciVI) that have at least one 5'-TpA sequence in their recognition sites. Weak inhibitions were found for the restriction enzymes EcoRI, SacI, BamHI, SalI, PstI and HindIII, which do not possess a 5'-TpA sequence at their restriction sites and the restriction site sequences of which consist of all bases, A, T, G and C. Trace or no inhibition was found for AscI and SmaI, the restriction site sequences of which are composed of only C and G. The results indicate the necessity of thymine (adenine) for the photosensitized DNA-binding activity of Dehydroeffusol. A strong inhibition against SphI, which does not have a 5'-TpA sequence in the restriction sequence, indicates that there are possibly other binding sequence(s) for Dehydroeffusol. With juncusol and UVA, strong inhibitions for KpnI and BciVI and trace inhibitions for PacI, XbaI, PmeI and DraI were found. This result also showed a preference of juncusol for 5'-TpA, but the preference could be more selective than that of Dehydroeffusol depending on the surrounding sequences of 5'-TpA in the respective restriction sites. A strong inhibition of SphI by juncusol with UVA also indicated the existence of an unknown binding sequence for this compound. Generally, the DNA-binding activity of this compound was weaker than that of Dehydroeffusol.


