Boc-Thr(tBu)-OHCAS# 13734-40-2 |
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13734-40-2 | SDF | Download SDF |
| PubChem ID | 7015770 | Appearance | Powder |
| Formula | C13H25NO5 | M.Wt | 275.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
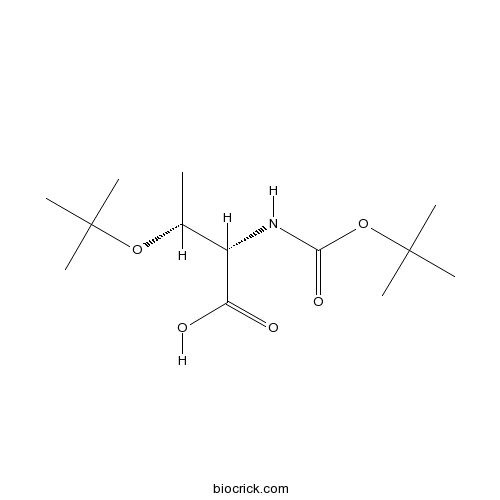
| Chemical Name | (2S,3R)-3-[(2-methylpropan-2-yl)oxy]-2-[(2-methylpropan-2-yl)oxycarbonylamino]butanoic acid | ||
| SMILES | CC(C(C(=O)O)NC(=O)OC(C)(C)C)OC(C)(C)C | ||
| Standard InChIKey | LKRXXARJBFBMCE-BDAKNGLRSA-N | ||
| Standard InChI | InChI=1S/C13H25NO5/c1-8(18-12(2,3)4)9(10(15)16)14-11(17)19-13(5,6)7/h8-9H,1-7H3,(H,14,17)(H,15,16)/t8-,9+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc-Thr(tBu)-OH Dilution Calculator

Boc-Thr(tBu)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6324 mL | 18.162 mL | 36.324 mL | 72.648 mL | 90.81 mL |
| 5 mM | 0.7265 mL | 3.6324 mL | 7.2648 mL | 14.5296 mL | 18.162 mL |
| 10 mM | 0.3632 mL | 1.8162 mL | 3.6324 mL | 7.2648 mL | 9.081 mL |
| 50 mM | 0.0726 mL | 0.3632 mL | 0.7265 mL | 1.453 mL | 1.8162 mL |
| 100 mM | 0.0363 mL | 0.1816 mL | 0.3632 mL | 0.7265 mL | 0.9081 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc-Thr(tBu)-OH
- Boc-Ser(tBu)-OH
Catalog No.:BCC3444
CAS No.:13734-38-8
- Boc-Sar-OH
Catalog No.:BCC3337
CAS No.:13734-36-6
- Boc-Phe-OH
Catalog No.:BCC3432
CAS No.:13734-34-4
- Boc-Lys-OH
Catalog No.:BCC3410
CAS No.:13734-28-6
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- LY 215840
Catalog No.:BCC7101
CAS No.:137328-52-0
- Cathepsin S inhibitor
Catalog No.:BCC1455
CAS No.:1373215-15-6
- Dehydroeffusol
Catalog No.:BCN2927
CAS No.:137319-34-7
- 4,5-Dioxo-4,5-seco-11(13)-cadinen-12-oic acid
Catalog No.:BCN1577
CAS No.:137288-61-0
- 4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pymol[2,3-d]pyrimodin-5-yl)ethyl]benzoic acid
Catalog No.:BCC8670
CAS No.:137281-39-1
- Pemetrexed
Catalog No.:BCC9115
CAS No.:137281-23-3
- Boc-Gln-OH
Catalog No.:BCC3382
CAS No.:13726-85-7
- Boc-Val-OH
Catalog No.:BCC3465
CAS No.:13734-41-3
- GSK J1
Catalog No.:BCC2231
CAS No.:1373422-53-7
- GSK J4 HCl
Catalog No.:BCC2230
CAS No.:1373423-53-0
- Nω-Propyl-L-arginine hydrochloride
Catalog No.:BCC6965
CAS No.:137361-05-8
- PF-5274857
Catalog No.:BCC3838
CAS No.:1373615-35-0
- Spathulatol
Catalog No.:BCN6877
CAS No.:1373888-27-7
- Diacerein
Catalog No.:BCN2291
CAS No.:13739-02-1
- 15,16-Epoxy-15-ethoxy-6beta,13-dihydroxylabd-8-en-7-one
Catalog No.:BCN7428
CAS No.:1374328-47-8
- LY 235959
Catalog No.:BCC6892
CAS No.:137433-06-8
- TUG 891
Catalog No.:BCC6235
CAS No.:1374516-07-0
- BRD4770
Catalog No.:BCC5525
CAS No.:1374601-40-7
- LEE011 succinate
Catalog No.:BCC4102
CAS No.:1374639-75-4
N alpha-trifluoroacetylation of N-terminal hydroxyamino acids: a new side reaction in peptide synthesis.[Pubmed:1450523]
Pept Res. 1992 Sep-Oct;5(5):287-92.
In the synthesis of the double-chain bis-cystinyl hinge fragment 225-232/225'-232' of the human IgG1, which contains two N-terminal threonine residues, the final acidolytic deprotection step, with 99% aqueous trifluoroacetic acid, was accompanied by formation of remarkable amounts of an unknown side product. This has been identified as the N alpha-mono-trifluoroacetylated product; however, even bis-trifluoroacetylation was found to occur by prolonged exposure of the parallel dimer to the reaction medium. Similarly, the model compounds H-Thr(tBu)-Phe-OH, [Boc-Thr(tBu)-Cys-OH]2 and Boc-Thr(tBu)-Cys(StBu)-Ala-OH were acylated by treatment with trifluoroacetic acid at rates that suggest a rather pronounced sequence dependency. Since, on the other hand, the model compound [Boc-Ala-Cys-OH]2 was not trifluoroacetylated at all under identical conditions, the reaction has to proceed prevalently via intermediate formation of the trifluoroacetyl ester of N-terminal hydroxyamino acids, followed by O-->N shift according to the hydroxyoxazolidine mechanism. The experimental data indicate also that under favored conditions aminolysis of the trifluoroacetyl ester via an inter- or intramolecular pathway may contribute to the overall reaction rate.
Synthesis and biological activity of some linear and cyclic kinin analogues.[Pubmed:7960398]
Int J Pept Protein Res. 1994 Jul;44(1):1-9.
Syntheses are described of some linear and cyclic kinin analogues. Cyclization, by the diphenyl-phosphorazide method, of linear peptides prepared by the solid-phase procedure based on Fmoc chemistry, was used for preparing cyclo-bradykinin and cyclo-kallidin (cyclo-Lys-bradykinin). Removal of the protecting group from the lysine side chain of cyclo-kallidin followed by acylation with the N-terminal sequence of vespulakinin 1 (VSK 1), Fmoc-Thr(tBu)-Ala-Thr(tBu)-Thr(tBu)-Arg(Pmc)-Arg(Pmc)-Gly-OH, by the Bop-HOBt procedure, yielded the protected N epsilon-(1-8 VSK 1)-cyclo-N alpha-kallidin, which was deblocked by acid treatment and purified by semi-preparative HPLC. The diglycosylated 1-8 VSK 1 sequence Boc-Thr(tBu)-Ala-(Gal beta)Thr-(Gal beta)Thr-Arg(Pmc)-Arg(Pmc)-Gly-OH was also synthesized by the solid-phase procedure and used to prepare the N epsilon-[(Gal beta)Thr3, (Gal beta)Thr4, 1-8 VSK 1]-cyclo-N-alpha- kallidin. Peptides and glycopeptides were characterized by amino acid analysis, optical rotation, analytical HPLC and FAB-MS. Preliminary pharmacological experiments showed that the cyclic kinin analogues are much less potent then bradykinin but still show specific bradykinin-like actions that support the hypothesis of the presence of a pharmacophore in the centre of the (brady)kinin molecule.


