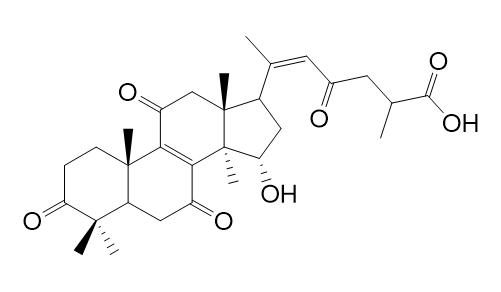
Ganoderenic acid GCAS# 120481-73-4 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 120481-73-4 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C30H40O7 | M.Wt | 512.6 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ganoderenic acid G Dilution Calculator

Ganoderenic acid G Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9508 mL | 9.7542 mL | 19.5084 mL | 39.0168 mL | 48.771 mL |
| 5 mM | 0.3902 mL | 1.9508 mL | 3.9017 mL | 7.8034 mL | 9.7542 mL |
| 10 mM | 0.1951 mL | 0.9754 mL | 1.9508 mL | 3.9017 mL | 4.8771 mL |
| 50 mM | 0.039 mL | 0.1951 mL | 0.3902 mL | 0.7803 mL | 0.9754 mL |
| 100 mM | 0.0195 mL | 0.0975 mL | 0.1951 mL | 0.3902 mL | 0.4877 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ganolucidic acid B
Catalog No.:BCX0012
CAS No.:98683-75-1
- 2-(2,4-Dihydroxyphenyl)-5,6-methylenedioxybenzofuran (ABF)
Catalog No.:BCX0011
CAS No.:67121-26-0
- 4-O-Coumaroylquinic acid
Catalog No.:BCX0010
CAS No.:53539-37-0
- Quercetin-3-O-[alpha-L-rhamnose-(1->2)-beta-D-glucopyranosyl]-5-O-beta-D-glucopyranoside
Catalog No.:BCX0009
CAS No.:1309795-36-5
- Ganoweberianic acid E
Catalog No.:BCX0008
CAS No.:1309931-90-5
- Massonianoside D
Catalog No.:BCX0007
CAS No.:85115-04-4
- beta-Hydroxyacteoside
Catalog No.:BCX0006
CAS No.:109279-13-2
- Isorhamnetin-3-O-rutinoside-7-O-glucoside
Catalog No.:BCX0005
CAS No.:55481-91-9
- Campneoside II
Catalog No.:BCX0004
CAS No.:95587-86-3
- Oxytroflavoside B
Catalog No.:BCX0003
CAS No.:1391144-81-2
- Kihadanin D
Catalog No.:BCX0002
CAS No.:2770024-83-2
- Quercetin 3,5-O-diglucoside
Catalog No.:BCX0001
CAS No.:206257-35-4
- 10-Deacetylcephalomannine
Catalog No.:BCX0014
CAS No.:76429-85-1
- Hosenkoside D
Catalog No.:BCX0015
CAS No.:156823-94-8
- Dihydroconfertin
Catalog No.:BCX0016
CAS No.:68832-40-6
- Regaloside I
Catalog No.:BCX0017
CAS No.:126239-78-9
- Herbacetin 3-sophoroside-8-glucoside
Catalog No.:BCX0018
CAS No.:77298-68-1
- erythro-Austrobailignan-6
Catalog No.:BCX0019
CAS No.:114127-24-1
- 12beta-Acetoxy-3,7,11,15,23-pentaoxo-lanost-8,20-dien-26-oic acid
Catalog No.:BCX0020
CAS No.:1309931-91-6
- Sophoraflavone A
Catalog No.:BCX0021
CAS No.:105594-08-9
- Ganoderic acid GS-3
Catalog No.:BCX0022
CAS No.:1206781-66-9
- 5-Hydroxy-4a,8-dimethyl-3-methylen-decahydroazuleno[6,5-b]furan-2(3H)-on
Catalog No.:BCX0023
CAS No.:114579-31-6
- Hainanic acid B
Catalog No.:BCX0024
CAS No.:1637737-46-2
- Kidjolanin
Catalog No.:BCX0025
CAS No.:38395-01-6
Network pharmacology analysis of the anti-cancer pharmacological mechanisms of Ganoderma lucidum extract with experimental support using Hepa1-6-bearing C57 BL/6 mice.[Pubmed:28882624]
J Ethnopharmacol. 2018 Jan 10;210:287-295.
ETHNOPHARMACOLOGICAL RELEVANCE: Ganoderma lucidum (GL) is an oriental medical fungus, which was used to prevent and treat many diseases. Previously, the effective compounds of Ganoderma lucidum extract (GLE) were extracted from two kinds of GL, [Ganoderma lucidum (Leyss. Ex Fr.) Karst.] and [Ganoderma sinense Zhao, Xu et Zhang], which have been used for adjuvant anti-cancer clinical therapy for more than 20 years. However, its concrete active compounds and its regulation mechanisms on tumor are unclear. AIM OF THE STUDY: In this study, we aimed to identify the main active compounds from GLE and to investigate its anti-cancer mechanisms via drug-target biological network construction and prediction. MATERIALS AND METHODS: The main active compounds of GLE were identified by HPLC, EI-MS and NMR, and the compounds related targets were predicted using docking program. To investigate the functions of GL holistically, the active compounds of GL and related targets were predicted based on four public databases. Subsequently, the Identified-Compound-Target network and Predicted-Compound-Target network were constructed respectively, and they were overlapped to detect the hub potential targets in both networks. Furthermore, the qRT-PCR and western-blot assays were used to validate the expression levels of target genes in GLE treated Hepa1-6-bearing C57 BL/6 mice. RESULTS: In our work, 12 active compounds of GLE were identified, including Ganoderic acid A, Ganoderenic acid A, Ganoderic acid B, Ganoderic acid H, Ganoderic acid C2, Ganoderenic acid D, Ganoderic acid D, Ganoderenic acid G, Ganoderic acid Y, Kaemferol, Genistein and Ergosterol. Using the docking program, 20 targets were mapped to 12 compounds of GLE. Furthermore, 122 effective active compounds of GL and 116 targets were holistically predicted using public databases. Compare with the Identified-Compound-Target network and Predicted-Compound-Target network, 6 hub targets were screened, including AR, CHRM2, ESR1, NR3C1, NR3C2 and PGR, which was considered as potential markers and might play important roles in the process of GLE treatment. GLE effectively inhibited tumor growth in Hepa1-6-bearing C57 BL/6 mice. Finally, consistent with the results of qRT-PCR data, the results of western-blot assay demonstrated the expression levels of PGR and ESR1 were up-regulated, as well as the expression levels of NR3C2 and AR were down-regulated, while the change of NR3C1 and CHRM2 had no statistical significance. CONCLUSIONS: The results indicated that these 4 hub target genes, including NR3C2, AR, ESR1 and PGR, might act as potential markers to evaluate the curative effect of GLE treatment in tumor. And, the combined data provide preliminary study of the pharmacological mechanisms of GLE, which may be a promising potential therapeutic and chemopreventative candidate for anti-cancer.


