HancinoneCAS# 104013-61-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104013-61-8 | SDF | Download SDF |
| PubChem ID | 101370414 | Appearance | Powder |
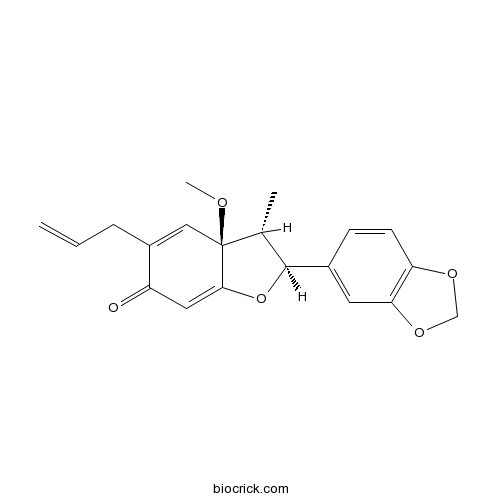
| Formula | C20H20O5 | M.Wt | 340.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3R,3aS)-2-(1,3-benzodioxol-5-yl)-3a-methoxy-3-methyl-5-prop-2-enyl-2,3-dihydro-1-benzofuran-6-one | ||
| SMILES | CC1C(OC2=CC(=O)C(=CC12OC)CC=C)C3=CC4=C(C=C3)OCO4 | ||
| Standard InChIKey | GGRIWHJBFXFKGS-CFGAKRJMSA-N | ||
| Standard InChI | InChI=1S/C20H20O5/c1-4-5-14-10-20(22-3)12(2)19(25-18(20)9-15(14)21)13-6-7-16-17(8-13)24-11-23-16/h4,6-10,12,19H,1,5,11H2,2-3H3/t12-,19+,20+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Hancinone Dilution Calculator

Hancinone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9377 mL | 14.6886 mL | 29.3772 mL | 58.7544 mL | 73.443 mL |
| 5 mM | 0.5875 mL | 2.9377 mL | 5.8754 mL | 11.7509 mL | 14.6886 mL |
| 10 mM | 0.2938 mL | 1.4689 mL | 2.9377 mL | 5.8754 mL | 7.3443 mL |
| 50 mM | 0.0588 mL | 0.2938 mL | 0.5875 mL | 1.1751 mL | 1.4689 mL |
| 100 mM | 0.0294 mL | 0.1469 mL | 0.2938 mL | 0.5875 mL | 0.7344 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Icariside E4
Catalog No.:BCN9693
CAS No.:126253-42-7
- Murrayamine O
Catalog No.:BCN9692
CAS No.:166990-10-9
- Methyl lucidenate L
Catalog No.:BCN9691
CAS No.:110267-46-4
- 1,1'-bislusianthridin
Catalog No.:BCN9690
CAS No.:182296-65-7
- Jasamplexoside A
Catalog No.:BCN9689
CAS No.:147764-93-0
- Triandrin
Catalog No.:BCN9688
CAS No.:19764-35-3
- Jasamplexoside C
Catalog No.:BCN9687
CAS No.:147742-02-7
- 3β-Hydroxycinnamolide
Catalog No.:BCN9686
CAS No.:124987-03-7
- Periplocogenin
Catalog No.:BCN9685
CAS No.:112899-63-5
- Kmeriol
Catalog No.:BCN9684
CAS No.:54306-10-4
- 7-Ketoisodrimenin
Catalog No.:BCN9683
CAS No.:73036-54-1
- Glabratine
Catalog No.:BCN9682
CAS No.:142750-47-8
- 1,5-Dihydroxy-6,7-dimethoxyxanthone
Catalog No.:BCN9695
CAS No.:38710-31-5
- 14-O-Acetylneoline
Catalog No.:BCN9696
CAS No.:1354-86-5
- Futoamide
Catalog No.:BCN9697
CAS No.:23477-80-7
- Anhydroscandenolide
Catalog No.:BCN9698
CAS No.:114742-71-1
- 2-Hydroxy-1-methoxyxanthone
Catalog No.:BCN9699
CAS No.:16302-46-8
- 14-Benzoyl-8-O-methylaconine
Catalog No.:BCN9700
CAS No.:93772-68-0
- Adenostemmoic acid E
Catalog No.:BCN9701
CAS No.:130217-22-0
- Gancaonin J
Catalog No.:BCN9702
CAS No.:129280-37-1
- Indole-3-carboxylic acid β-D-glucopyranosyl ester
Catalog No.:BCN9703
CAS No.:106871-55-0
- Adenostemmoic acid G
Catalog No.:BCN9704
CAS No.:130217-26-4
- 12-O-Methylinophyllum D
Catalog No.:BCN9705
CAS No.:40883-10-1
- Onjixanthone I
Catalog No.:BCN9706
CAS No.:136083-92-6
A Network Pharmacology-Based Strategy For Predicting Active Ingredients And Potential Targets Of LiuWei DiHuang Pill In Treating Type 2 Diabetes Mellitus.[Pubmed:31819371]
Drug Des Devel Ther. 2019 Nov 28;13:3989-4005.
Background: Traditional Chinese medicine (TCM) formulations have proven to be advantageous in clinical treatment and prevention of disease. LiuWei DiHuang Pill (LWDH Pill) is a TCM that was employed to treat type 2 diabetes mellitus (T2DM). However, a holistic network pharmacology approach to understanding the active ingredients and the therapeutic mechanisms underlying T2DM has not been pursued. Methods: A network pharmacology approach including drug-likeness evaluation, oral bioavailability prediction, virtual docking, and network analysis has been used to predict the active ingredients and potential targets of LWDH Pill in the treatment of type 2 diabetes. Results: The comprehensive network pharmacology approach was successfully to identify 45 active ingredients in LWDH Pill. 45 active ingredients hit by 163 potential targets related to T2DM. Ten of the more highly predictive components (such as :quercetin, Kaempferol, Stigmasterol, beta-sitosterol, Kadsurenone, Diosgenin, Hancinone C, Hederagenin, Garcinone B, Isofucosterol) are involved in anti-inflammatory, anti-oxidative stress, and the reduction of beta cell damage. LWDH Pill may play a role in the treatment of T2DM and its complications (atherosclerosis and nephropathy) through the AGE-RAGE signaling pathway, TNF signaling pathway, and NF-kappa B signaling pathway. Conclusion: Based on a systematic network pharmacology approach, our works successfully predict the active ingredients and potential targets of LWDH Pill for application to T2DM and helps to illustrate mechanism of action on a comprehensive level. This study provides identify key genes and pathway associated with the prognosis and pathogenesis of T2DM from new insights, which also demonstrates a feasible method for the research of chemical basis and pharmacology in LWDH Pill.
[Neolignans and lignan from Piper wallichii].[Pubmed:20394289]
Zhongguo Zhong Yao Za Zhi. 2010 Jan;35(2):180-2.
To investigate the chemical constituents of the aerial part of Piper wallichii. Nine compounds were isolated by various chromatographic techniques and the structures were elucidated by their physicochemical properties and the spectral data analysis. Nine compounds were identified as one lignan (-)-galbelgin (1) and eight neolignans: denudatin B (2), Hancinone D (3), (+)-licarin A (4), kadsurenone (5), wallichinine (6), Hancinone C (7), Hancinone B (8), (+)-burchellin (9). Compounds 1, 3, 4, 8, 9 were isolated from this plant for the first time.
[Neolignans from Piper polysyphorum C.DC].[Pubmed:1957684]
Yao Xue Xue Bao. 1991;26(5):345-50.
Piper polysyphorum C.DC (Piperaceae) is indigenous to the southern part of China. In the course of screening for inhibitors of platelet activating factor (PAF), the nonpolar fraction was found to exhibit PAF inhibitory activity. One new neclignan named polysyphorin and three new enantiomeric forms of (+)-virolongin, (+)-grandisin and (+)-lancifolin D were isolated. Two known neolignans, wallichinine and Hancinone D were also obtained from the same sources. The structure determination was based upon spectroscopic analysis (UV, IR, CD, MS, 1HNMR, 13CNMR, COSY) and derivative preparation. The structure for polysyphorin was established as threo-delta 7'-7-hydroxy-3,4,5,3',5'-pentamethoxy-8-O-4' -neolignan. It is a racemic enantiomer. The PAF inhibitory activities were reported.
[The isolation and identification of PAF inhibitors from Piper wallichii (Miq.) Hand-Mazz and P. hancei Maxim].[Pubmed:2609983]
Yao Xue Xue Bao. 1989;24(6):438-43.
Platelet activating factor (PAF) is a highly potent endogenous phospholipid mediator, involved in various inflammatory and cardiovascular disorders. As part of a research program dealing with PAF inhibitors isolated from Piper plant species, we have isolated kadsurenone (I), denudatin B (II), and N-isobutyl-deca-trans-2-trans-4-dienamide (III) from Piped wallichii (Miq.) Hand-Mazz. and P. hancei Maxim. In a continuing search for potential PAF inhibitor from plants, using PAF induced platelet aggregation as a guide, a new neolignan named Hancinone D (IV) was isolated from P. hancei maxim. By X-ray analysis it was identified as a racemate. The X-ray analysis led to a revision of the previously made structure assignment of Hancinone C. Another new neolignan named wallichinine (V), which was identified as an analogue of (IV), along with the known compounds Hancinone C (VI), galgravin (VII), dihydropiperlonguminine (VIII) and crotepoxide (IX) were isolated from P. wallichii (Miq.) Hand-Mazz. The structure determination was based upon spectroscopic analysis. All of the compounds were for the first time obtained from both plants. In the test of platelet aggregation caused by PAF, I, II, V, VI, VII showed inhibitory activity, whereas III, IV, VII, IX showed no activity.


