TriandrinCAS# 19764-35-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19764-35-3 | SDF | Download SDF |
| PubChem ID | 14048613 | Appearance | Powder |
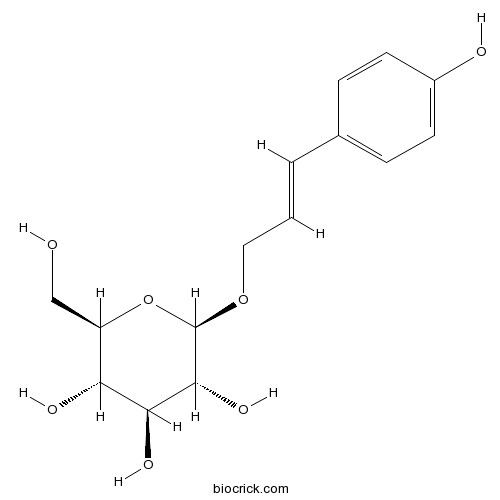
| Formula | C15H20O7 | M.Wt | 312.31 |
| Type of Compound | Phenylpropanoid | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-[(E)-3-(4-hydroxyphenyl)prop-2-enoxy]oxane-3,4,5-triol | ||
| SMILES | C1=CC(=CC=C1C=CCOC2C(C(C(C(O2)CO)O)O)O)O | ||
| Standard InChIKey | CNNXMGXBAZQZDE-HHMSBIESSA-N | ||
| Standard InChI | InChI=1S/C15H20O7/c16-8-11-12(18)13(19)14(20)15(22-11)21-7-1-2-9-3-5-10(17)6-4-9/h1-6,11-20H,7-8H2/b2-1+/t11-,12-,13+,14-,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Triandrin Dilution Calculator

Triandrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2019 mL | 16.0097 mL | 32.0195 mL | 64.0389 mL | 80.0487 mL |
| 5 mM | 0.6404 mL | 3.2019 mL | 6.4039 mL | 12.8078 mL | 16.0097 mL |
| 10 mM | 0.3202 mL | 1.601 mL | 3.2019 mL | 6.4039 mL | 8.0049 mL |
| 50 mM | 0.064 mL | 0.3202 mL | 0.6404 mL | 1.2808 mL | 1.601 mL |
| 100 mM | 0.032 mL | 0.1601 mL | 0.3202 mL | 0.6404 mL | 0.8005 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Jasamplexoside C
Catalog No.:BCN9687
CAS No.:147742-02-7
- 3β-Hydroxycinnamolide
Catalog No.:BCN9686
CAS No.:124987-03-7
- Periplocogenin
Catalog No.:BCN9685
CAS No.:112899-63-5
- Kmeriol
Catalog No.:BCN9684
CAS No.:54306-10-4
- 7-Ketoisodrimenin
Catalog No.:BCN9683
CAS No.:73036-54-1
- Glabratine
Catalog No.:BCN9682
CAS No.:142750-47-8
- Integracin B
Catalog No.:BCN9681
CAS No.:224186-05-4
- Muricarpone B
Catalog No.:BCN9680
CAS No.:886226-15-9
- Spirotryprostatin A
Catalog No.:BCN9679
CAS No.:182234-25-9
- Jaslanceoside B
Catalog No.:BCN9678
CAS No.:188300-82-5
- 10-Epiteuclatriol
Catalog No.:BCN9677
CAS No.:151563-36-9
- 8-Methoxyfissistigine C
Catalog No.:BCN9676
CAS No.:20824-18-4
- Jasamplexoside A
Catalog No.:BCN9689
CAS No.:147764-93-0
- 1,1'-bislusianthridin
Catalog No.:BCN9690
CAS No.:182296-65-7
- Methyl lucidenate L
Catalog No.:BCN9691
CAS No.:110267-46-4
- Murrayamine O
Catalog No.:BCN9692
CAS No.:166990-10-9
- Icariside E4
Catalog No.:BCN9693
CAS No.:126253-42-7
- Hancinone
Catalog No.:BCN9694
CAS No.:104013-61-8
- 1,5-Dihydroxy-6,7-dimethoxyxanthone
Catalog No.:BCN9695
CAS No.:38710-31-5
- 14-O-Acetylneoline
Catalog No.:BCN9696
CAS No.:1354-86-5
- Futoamide
Catalog No.:BCN9697
CAS No.:23477-80-7
- Anhydroscandenolide
Catalog No.:BCN9698
CAS No.:114742-71-1
- 2-Hydroxy-1-methoxyxanthone
Catalog No.:BCN9699
CAS No.:16302-46-8
- 14-Benzoyl-8-O-methylaconine
Catalog No.:BCN9700
CAS No.:93772-68-0
Phenolic Compounds from Populus alba L. and Salix subserrata Willd. (Salicaceae) Counteract Oxidative Stress in Caenorhabditis elegans.[Pubmed:31137712]
Molecules. 2019 May 24;24(10). pii: molecules24101999.
Utilizing bioassay- and TLC-guided column chromatography, fifteen secondary metabolites from Populus alba and eight compounds from Salix subserrata were isolated, including a novel plant metabolite salicyl ether and characterized using ultralviolet light (UV) absorbance, mass spectrometry (MS), (1)H-, (13)C-NMR (nuclear magnetic resonance), heteronuclear single quantum coherence spectroscopy (HSQC) and heteronuclear multiple bond correlation (HMBC). The extracts, their sub-fractions and the isolated compounds exhibited promising antioxidant activities in vitro in DPPH and FRAP assays. Also, the extracts of P. alba leaf (PL), shoots (PS), and S. subserrata leaf (SL) demonstrated substantial antioxidant activities in vivo in the multicellular model organism Caenorhabditis elegans. For the first time, the isolated secondary metabolites, aromadendrin, tremuloidin, salicin, isorhamnetin-3-O-beta-d-rutinoside, gallocatechin, Triandrin, and chrysoeriol-7-O-glucuronide were investigated. They exhibited substantial antioxidant activities in vivo. Salicin, isorhamnetin-3-O-beta-d-rutinoside and gallocatechin, in particular, protected the worms against a lethal dose of the pro-oxidant juglone (80 microM), decreased the endogenous reactive oxygen species (ROS) level to 45.34%, 47.31%, 68.09% and reduced juglone- induced hsp-16.2::GFP (green fluorescence protein) expression to 79.62%, 70.17%, 26.77%, respectively. However, only gallocatechin induced higher levels of sod-3 expression. These findings support the traditional use of Populus alba and Salix subserrata for treating inflammation especially when ROS are involved.
Phenylalkanoid Glycosides (Non-Salicinoids) from Wood Chips of Salix triandra x dasyclados Hybrid Willow.[Pubmed:30909533]
Molecules. 2019 Mar 23;24(6). pii: molecules24061152.
Salix triandra (almond leaved willow) is an established crop, grown in coppicing regimes for basket-making materials. It is known as a source of non-salicinoid phenolic glycosides, such as Triandrin and salidroside. A spontaneous natural hybrid of S. triandra and S. dasyclados was subjected to metabolite profiling by high resolution LC-MS, and 22 phenolic glycosides, including 18 that are new to the Salicaceae, were identified. Structures were determined by HPLC isolation and NMR methods. The hybridisation process has introduced novel chemistry into the Salix phenolic glycoside palette, in particular, the ability to generate disaccharide conjugates where the glycosyl group is further extended by a range of sugars, including apiose, rhamnose, xylose, and arabinose. Also of note is the appearance of chavicol derivatives, also not previously seen in Salix spp. The work demonstrates the plasticity of the phenolic glycoside biosynthetic pathway, and the potential to improve established crops such as S. triandra and S. dasyclados, via high-value metabolites, for both basketry and bioenergy markets.
Metabolite Profile and Antiproliferative Effects in HaCaT Cells of a Salix reticulata Extract.[Pubmed:28449181]
Planta Med. 2017 Oct;83(14-15):1149-1158.
Phenolic constituents of Salix reticulata (Salicaceae) and antiproliferative activity of an extract and individual compounds were investigated in immortalized human non-tumorigenic keratinocytes (HaCaT). A MeOH extract from aerial parts afforded several flavonoids, including luteolin and apigenin glycosides (2-5 and 9) and catechin (1), two procyanidin fractions, and the phenolic glucosides picein (6), Triandrin (7), and salicortin (8). In an adenosine triphosphate assay, the MeOH extract reduced cell viability by approximately 60 % at a concentration of 100 microg/mL. Cell proliferation was assessed with a BrdU incorporation ELISA assay. The extract inhibited proliferation of HaCaT cells in a concentration-dependent manner, with approximately 50 % inhibition at 100 microg/mL. In time-lapse assays, the extract showed distinct inhibitory effects on cell migration at concentrations of 12.5, 25, and 50 microg/mL. The activity of selected constituents was also determined. Luteolin-7-O-beta-glucuronide (3) significantly inhibited cell proliferation at concentrations of 10 and 50 microM. In contrast, luteolin-7-O-beta-glucopyranoside (2) and a procyanidin fraction (P1) had only weak effects, while picein (6) and salicortin (8) did not affect cell proliferation. Luteolin-7-O-beta-glucuronide (10 microM) and, to a lesser extent, the procyanidin fraction (10 microg/mL) also inhibited cell migration.
The vegetative buds of Salix myrsinifolia are responsive to elevated UV-B and temperature.[Pubmed:25749271]
Plant Physiol Biochem. 2015 Aug;93:66-73.
The predicted rise in temperature and variable changes in ultraviolet-B radiation will have marked effects on plant growth and metabolism. Different vegetative parts of trees have been studied to detect the impacts of enhanced temperature and UV-B, but the effects on buds have rarely been considered. In the present study, Salix myrsinifolia clones were subjected to enhanced UV-B and temperature over two growing seasons starting from 2009, and measured springtime bud development and concentrations of phenolic compounds. In 2010 and 2011 the buds under increased temperature were up to 30% longer than those in control plots. On the other hand, UV-B combined with elevated temperature significantly decreased bud length by 4-5% in 2010. This effect was stronger in males than in females. The vegetative buds contained high constitutive amounts of chlorogenic acid derivatives, which may explain the weak increase in hyperin and chlorogenic acid that are usual UV-B sheltering compounds. The elevated temperature treatment significantly increased salicin content (about 18% in males and 22% in females), while Triandrin concentration decreased by only 50% in females. Our results indicate that vegetative bud size is highly affected by seasonal temperature, while UV-B induced a weaker and transient effect.
Mechanism of action of Rhodiola, salidroside, tyrosol and triandrin in isolated neuroglial cells: an interactive pathway analysis of the downstream effects using RNA microarray data.[Pubmed:25172797]
Phytomedicine. 2014 Sep 25;21(11):1325-48.
AIM: The aim of this study was to identify the targets (genes, interactive signaling pathways, and molecular networks) of Rhodiola rosea extract in isolated neuroglia cells and to predict the effects of Rhodiola extract on cellular functions and diseases. In addition, the potential mechanism of action of Rhodiola rosea extract was elucidated, and the "active principle" among the three isolated constituents (salidroside, Triandrin, and tyrosol) was identified. METHODS: Gene expression profiling was performed using the T98G human neuroglia cell line after treatment with the Rhodiola rosea SHR-5 extract and several of its individual constituents (salidroside, Triandrin and tyrosol). An interactive pathway analysis of the downstream effects was performed using datasets containing significantly up- and down-regulated genes, and the effects on cellular functions and diseases were predicted. RESULTS: In total, the expression of 1062 genes was deregulated by the Rhodiola extract (631 analyzed, 336 - up-regulated, 295 - down-regulated), and 1052, 1062, and 1057 genes were deregulated by salidroside, Triandrin, and tyrosol, respectively. The analysis of the downstream effects shows that the most significant effects of Rhodiola are associated with cardiovascular (72 deregulated genes), metabolic (63 genes), gastrointestinal (163 genes), neurological (95 genes), endocrine (60 genes), behavioral (50 genes), and psychological disorders (62 genes). The most significantly affected canonical pathways across the entire dataset, which contains the 1062 genes deregulated by Rhodiola, were the following: (a) communication between innate and adaptive immune cells, (b) eNOS signaling, (c) altered T and B cell signaling in rheumatoid arthritis, (d) axonal guidance signaling, (e) G-protein coupled receptor signaling, (f) glutamate receptor signaling, (g) ephrin receptor signaling, (h) cAMP-mediated, and (i) atherosclerosis signaling pathways. Genes associated with behavior and behavioral diseases were identified within intracellular signaling pathways (d) through (h). The analysis of the downstream effects predicted decreases in emotional and aggressive behavior, which corroborates the results from preclinical and clinical studies of the use of Rhodiola for the treatment of depression and anxiety. Of the 17 genes that regulate emotional behavior, nine exhibit expression patterns that are consistent with decreases in emotional behavior (z-score -2.529), and all five relevant genes are expressed in a manner consistent with decreases in aggressive behavior (z-score -2.197). A decrease in seizures and infarct sizes and an increase in the chemotaxis of cells were predicted to accompany the decrease in emotional and aggressive behaviors. CONCLUSIONS: Rhodiola exhibits a multi-targeted effect on transcription to regulate the cellular response, affecting the various signaling pathways and molecular networks associated with beneficial effects on emotional behavior, particularly aggressive behavior, and with psychological, neurological, cardiovascular, metabolic, endocrine, and gastrointestinal disorders. Each of the purified compounds has its own pharmacological profile, which is both similar to and different from that of the total Rhodiola extract. In general, several compounds contribute to the specific cellular or/and physiological function of the extract in various diseases.
Synergy and Antagonism of Active Constituents of ADAPT-232 on Transcriptional Level of Metabolic Regulation of Isolated Neuroglial Cells.[Pubmed:23430930]
Front Neurosci. 2013 Feb 20;7:16.
Gene expression profiling was performed on the human neuroglial cell line T98G after treatment with adaptogen ADAPT-232 and its constituents - extracts of Eleutherococcus senticosus root, Schisandra chinensis berry, and Rhodiola rosea root as well as several constituents individually, namely, eleutheroside E, schizandrin B, salidroside, Triandrin, and tyrosol. A common feature for all tested adaptogens was their effect on G-protein-coupled receptor signaling pathways, i.e., cAMP, phospholipase C (PLC), and phosphatidylinositol signal transduction pathways. Adaptogens may reduce the cAMP level in brain cells by down-regulation of adenylate cyclase gene ADC2Y and up-regulation of phosphodiesterase gene PDE4D that is essential for energy homeostasis as well as for switching from catabolic to anabolic states and vice versa. Down-regulation of cAMP by adaptogens may decrease cAMP-dependent protein kinase A activity in various cells resulting in inhibition stress-induced catabolic transformations and saving of ATP for many ATP-dependant metabolic transformations. All tested adaptogens up-regulated the PLCB1 gene, which encodes phosphoinositide-specific PLC and phosphatidylinositol 3-kinases (PI3Ks), key players for the regulation of NF-kappaB-mediated defense responses. Other common targets of adaptogens included genes encoding ERalpha estrogen receptor (2.9-22.6 fold down-regulation), cholesterol ester transfer protein (5.1-10.6 fold down-regulation), heat shock protein Hsp70 (3.0-45.0 fold up-regulation), serpin peptidase inhibitor (neuroserpin), and 5-HT3 receptor of serotonin (2.2-6.6 fold down-regulation). These findings can be reconciled with the observed beneficial effects of adaptogens in behavioral, mental, and aging-associated disorders. Combining two or more active substances in one mixture significantly changes deregulated genes profiles: synergetic interactions result in activation of genes that none of the individual substances affected, while antagonistic interactions result in suppression some genes activated by individual substances. These interactions can have an influence on transcriptional control of metabolic regulation both on the cellular level and the level of the whole organism. Merging of deregulated genes array profiles and intracellular networks is specific to the new substance with unique pharmacological characteristics. Presumably, this phenomenon could be used to eliminate undesirable effects (e.g., toxic effects) and increase the selectivity of pharmacological intervention.
Chromatographic analysis of salicylic compounds in different species of the genus Salix.[Pubmed:17880029]
J Sep Sci. 2007 Nov;30(17):2958-66.
The separation of nine phenol glycosides--salicin, salicortin, 2'-acetylsalicortin, populin, tremulacin, salidroside, Triandrin, picein and helicin--by normal phase (NP), reversed phase (RP) HPLC techniques and a coupling of NP and RP monolithic silica columns was studied. Among the above nine compounds only five--salicin, populin, tremulacin, salidroside and Triandrin--were resolved in an NP system with a mobile phase comprising hexane/isopropanol/methanol (87:12:1, v/v/v). Optimized separation was performed with two coupled monolithic silica columns of different polarity (bare silica and RP-18). The method was applied to verify the presence of salicylic compounds and other phenolic derivatives in the bark of six species from the genus Salix, namely S. purpurea, S. daphnoides clone 1095, S. alba clone 1100, S. triandra, S. viminalis, and S. herbacea. Gradient elution with a mobile phase composed of acetonitrile and water containing 0.05% of trifluoroacetic acid, with increasing acetonitrile concentration from 3% to 48%, was chosen as optimal. For the selective detection of the salicylic compounds, an evaporative light scattering detector was employed along with a UV detector. The differences in the composition of phenols in the different plant materials were confirmed. Additionally, it must be emphasized that for the first time the presence of 2'-acetylsalicortin was revealed in S. alba clone 1100. Furthermore, an SPE-HPLC method was developed for the rapid analysis of the salicin content, analyzed as free and total fraction, in willow barks. The determined concentrations of total salicin varied from 25.4 mg/g in S. alba clone 1100 to 96.47 mg/g in S. daphnoides clone 1095.
HPLC-MS/MS analysis of willow bark extracts contained in pharmaceutical preparations.[Pubmed:16315493]
Phytochem Anal. 2005 Nov-Dec;16(6):470-8.
Preparations containing willow bark extract are popular herbal remedies, but they are mostly standardised with respect to only one compound (usually salicin). RP-HPLC using a C18-column eluted with water:methanol:tetrahydrofuran and coupled to electrospray triple-quadrupole MS and MS/MS was used for the characterisation of dried extracts of Salix spp. and for the identification of their constituents. Comparison with reference substances led to the identification of 13 compounds (saligenin, salicylic acid, salicin, isosalicin, picein, salidroside, Triandrin, salicoylsalicin, salicortin, isosalipurposide, salipurposide, naringenin-7-O-glucoside and tremulacin). Two pharmaceutical preparations containing willow bark extract, used in clinical trials and labelled Salix daphnoides and S. purpurea x daphnoides extracts, were compared using the described method and exhibited several clear differences, the most prominent of which was the possible presence of picein in the former preparation. The described method may be utilised for the characterisation of herbal medicines in order to ensure comparability of medication in further clinical trials.
Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration.[Pubmed:16261511]
Phytother Res. 2005 Oct;19(10):819-38.
Plant adaptogens are compounds that increase the ability of an organism to adapt to environmental factors and to avoid damage from such factors. The beneficial effects of multi-dose administration of adaptogens are mainly associated with the hypothalamic-pituitary-adrenal (HPA) axis, a part of the stress-system that is believed to play a primary role in the reactions of the body to repeated stress and adaptation. In contrast, the single dose application of adaptogens is important in situations that require a rapid response to tension or to a stressful situation. In this case, the effects of the adaptogens are associated with another part of the stress-system, namely, the sympatho-adrenal-system (SAS), that provides a rapid response mechanism mainly to control the acute reaction of the organism to a stressor. This review focuses primarily on the SAS-mediated stimulating effects of single doses of adaptogens derived from Rhodiola rosea, Schizandra chinensis and Eleutherococcus senticosus. The use of these drugs typically generates no side effects, unlike traditional stimulants that possess addiction, tolerance and abuse potential, produce a negative effect on sleep structure, and cause rebound hypersomnolence or 'come down' effects. Furthermore, single administration of these adaptogens effectively increases mental performance and physical working capacity in humans. R. rosea is the most active of the three plant adaptogens producing, within 30 min of administration, a stimulating effect that continues for at least 4-6 h. The active principles of the three plants that exhibit single dose stimulating effects are glycosides of phenylpropane- and phenylethane-based phenolic compounds such as salidroside, rosavin, syringin and Triandrin, the latter being the most active.


