InteriorinCAS# 119139-55-8 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

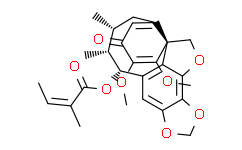
| Cas No. | 119139-55-8 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C27H30O8 | M.Wt | 482.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Interiorin Dilution Calculator

Interiorin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0725 mL | 10.3623 mL | 20.7245 mL | 41.4491 mL | 51.8113 mL |
| 5 mM | 0.4145 mL | 2.0725 mL | 4.1449 mL | 8.2898 mL | 10.3623 mL |
| 10 mM | 0.2072 mL | 1.0362 mL | 2.0725 mL | 4.1449 mL | 5.1811 mL |
| 50 mM | 0.0414 mL | 0.2072 mL | 0.4145 mL | 0.829 mL | 1.0362 mL |
| 100 mM | 0.0207 mL | 0.1036 mL | 0.2072 mL | 0.4145 mL | 0.5181 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tenacissoside E
Catalog No.:BCX0858
CAS No.:107347-56-8
- Tenacissoside B
Catalog No.:BCX0857
CAS No.:107424-13-5
- Tenacissoside D
Catalog No.:BCX0856
CAS No.:107347-57-9
- Tenacissoside C
Catalog No.:BCX0855
CAS No.:107347-58-0
- Okicamelliaside
Catalog No.:BCX0854
CAS No.:949148-45-2
- Aloinoside A
Catalog No.:BCX0853
CAS No.:56645-88-6
- Tomentosin
Catalog No.:BCX0852
CAS No.:33649-15-9
- Aloinoside B
Catalog No.:BCX0851
CAS No.:11006-91-0
- Gymnoside I
Catalog No.:BCX0850
CAS No.:899430-01-4
- Pseudoginsenoside Rg3(E)
Catalog No.:BCX0849
CAS No.:1012886-99-5
- 6,7-Dihydroxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone
Catalog No.:BCX0848
CAS No.:626236-07-5
- Annatto
Catalog No.:BCX0847
CAS No.:1393-63-1
- 6''-Malonylthymosin I
Catalog No.:BCX0860
CAS No.:528606-92-0
- Dieugenol
Catalog No.:BCX0861
CAS No.:4433-08-3
- (+)-5-methoxydeydrodiisoeugenol
Catalog No.:BCX0862
CAS No.:1967042-42-7
- Eucomoside B
Catalog No.:BCX0863
CAS No.:951672-66-5
- Polyporusterone C
Catalog No.:BCX0864
CAS No.:141360-90-9
- Crocin E
Catalog No.:BCX0865
CAS No.:58050-17-2
- Acetyl Dopamine DimerIV
Catalog No.:BCX0866
CAS No.:1632326-65-8
- Deacetylkadsurin
Catalog No.:BCX0867
CAS No.:51670-42-9
- α-Curcumene
Catalog No.:BCX0868
CAS No.:644-30-4
- Acetyl Dopamine DimerIII
Catalog No.:BCX0869
CAS No.:916888-49-8
- Lugrandoside
Catalog No.:BCX0870
CAS No.:117457-37-1
- N-acetyldopamine dimmers A
Catalog No.:BCX0871
CAS No.:1519015-73-6
Comparison of blood tonic efficacy and chemical constituents of Kadsura interior A.C. Smith and its closely related species.[Pubmed:35039063]
Chin Med. 2022 Jan 17;17(1):14.
BACKGROUND: The stems of Kadsura interior A. C. Smith are used as traditional Chinese medicine (TCM) Kadsurae Caulis, with the traditional efficacy of tonifying and invigorating the blood, therefore being favored to treat blood deficiency (BD) widely. However, the stems of K. interior and its closely related species are morphologically similar and they may readily be misused as Kadsurae Caulis, thus likely to exert negative effects on clinical efficacy and clinical medication safety. METHODS: Firstly, blood tonic efficacies of the stems of K. interior (KIS) and its closely related species were compared using BD mouse model induced by 1-acetyl-2-phenylhydrazine (APH) and cyclophosphamide (CTX). Secondly, the chemical constituents from the stems of K. interior and its closely related species were evaluated and compared using a plant metabolomics approach. Plant metabolomics in this study aims at discovering differential metabolites and comprehensively assessing the chemical constituents by combining state-of-the-art high-resolution UPLC-Q/TOF-MS/MS technique and multivariate data analysis. Finally, based on the pharmacological data and the chemical constituents in UPLC-Q/TOF-MS fingerprints, the potential blood tonic active markers were screened by the spectrum-effect relationship analysis and quantified by UPLC-UV-DAD. RESULTS: The ethanol extract of the stems of K. interior significantly increased the levels of hematocrit (HCT), hemoglobin (HGB), and red blood cells (RBC) in BD mice. In addition, it significantly increased the serum levels of interleukin 3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and macrophage-stimulating factor (M-CSF) in BD mice (P < 0.01). The blood tonic efficacy of the stems of K. interior was superior to those of its closely related species, especially at the dose of 200 mg/kg. Six differential compounds in the stems of K. interior were screened out to distinguish it from its closely related species. In combination with the results of the spectrum-effect relationship analysis, heteroclitin D, Interiorin C, and heteroclitin G were identified as potential bioactive markers. The contents of heteroclitin D and heteroclitin G in the freeze-dried powder of KIS were 15.90 and 3.74 mug/mg. CONCLUSIONS: This study illustrated the differences in the blood tonic efficacies and the chemical constituents of the stems of K. interior and its closely related species, and pinpointed the potential bioactive markers of K. interior.
Kadsutherin D, a new dibenzocyclooctadiene lignan from Kadsura species.[Pubmed:19023792]
Nat Prod Res. 2008;22(15):1344-9.
A new dibenzocyclooctadiene lignan, kadsutherin D (1), together with 12 known lignans, kadsurin (2), heteroclitin C (3), interiotherin C (4), schisanlignone A (5), binankadsurin A (6), angeloyl binankadsurin A (7), gomisin U (8), heteroclitin D (9), Interiorin (10), schiarisanrin C (11), heteroclitin G (12) and meso-dihydroguaiaretic acid (13) were isolated from the stems of Kadsura species. Their structures were elucidated by spectroscopic methods including 2D-NMR techniques.
Heteroclitin H, a new lignan from Kadsura heteroclita.[Pubmed:17135051]
J Asian Nat Prod Res. 2006 Oct-Nov;8(7):643-8.
A new dibenzocyclooctadiene lignan, heteroclitin H (1), was isolated together with seven known compounds, heteroclitin D (2), Interiorin B (3), Interiorin (4), neokasuranin (5), interiotherin C (6), gomisin J (7) and (+)-anwulignan (8), from the stems of Kadsura heteroclita. Their structures were elucidated by spectroscopic methods. This is the first report of the isolation of compounds 3, 5, 6, 7 and 8 from K. heteroclita.
Interiotherins C and D, two new lignans from Kadsura interior and antitumor-promoting effects of related neolignans on Epstein-Barr virus activation.[Pubmed:12350139]
J Nat Prod. 2002 Sep;65(9):1242-5.
Two new lignans, interiotherins C (1) and D (2), together with the known compounds Interiorin (3), heteroclitin F (4), neokadsuranin (5), heteroclitin D (6), kadsurin (7), gomisin A (8), schisandrin C (9), interiotherin A (10), angeloylgomisin R (11), gomisin G (12), interiotherin B (13), and gomisin C (14), were isolated from the stems of Kadsura interior. The structures and stereochemistries of the new compounds were determined from mass, CD, and NMR spectral data. Fourteen neolignans were screened as potential antitumor promoters by examining their ability to inhibit Epstein-Barr virus early antigen (EBV-EA) activation (induced by 12-O-tetradecanoylphorbol-13-acetate) in Raji cells. Neokadsuranin (5) and schisandrin C (9) were the most potent compounds. These data suggest that some neolignans might be valuable antitumor promoters or chemopreventors.
[Interiorin: a neolignan with a new skeleton from Kadsura interior].[Pubmed:17265308]
Planta Med. 1988 Oct;54(5):440-3.
From the aerial parts of KADSURA INTERIOR A. C. Sm. (Schizan-draceae) a neolignan, Interiorin ( 1), with a new skeleton was isolated. Its structure was elucidated by spectroscopic methods and by single X-ray diffraction analysis.


