OkicamelliasideCAS# 949148-45-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 949148-45-2 | SDF | Download SDF |
| PubChem ID | 10027940.0 | Appearance | Powder |
| Formula | C21H16O13 | M.Wt | 476.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
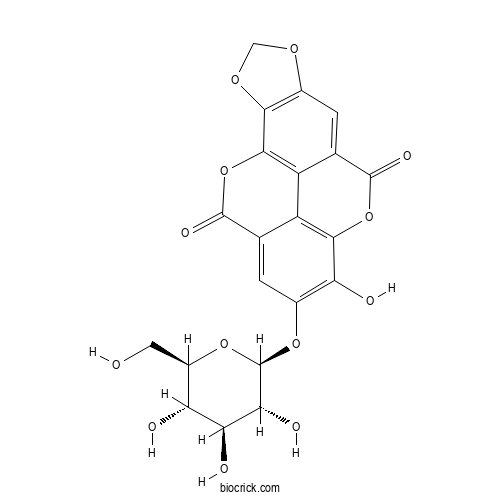
| Chemical Name | 12-hydroxy-13-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,5,10,17-tetraoxapentacyclo[9.6.2.02,6.08,18.015,19]nonadeca-1(18),2(6),7,11(19),12,14-hexaene-9,16-dione | ||
| SMILES | C1OC2=C(O1)C3=C4C(=C2)C(=O)OC5=C4C(=CC(=C5O)OC6C(C(C(C(O6)CO)O)O)O)C(=O)O3 | ||
| Standard InChIKey | IDAFRNDKIRARME-OZJCBLQYSA-N | ||
| Standard InChI | InChI=1S/C21H16O13/c22-3-9-12(23)14(25)15(26)21(32-9)31-7-1-5-10-11-6(19(27)33-17(10)13(7)24)2-8-16(30-4-29-8)18(11)34-20(5)28/h1-2,9,12,14-15,21-26H,3-4H2/t9-,12-,14+,15-,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Okicamelliaside Dilution Calculator

Okicamelliaside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0993 mL | 10.4967 mL | 20.9934 mL | 41.9868 mL | 52.4835 mL |
| 5 mM | 0.4199 mL | 2.0993 mL | 4.1987 mL | 8.3974 mL | 10.4967 mL |
| 10 mM | 0.2099 mL | 1.0497 mL | 2.0993 mL | 4.1987 mL | 5.2484 mL |
| 50 mM | 0.042 mL | 0.2099 mL | 0.4199 mL | 0.8397 mL | 1.0497 mL |
| 100 mM | 0.021 mL | 0.105 mL | 0.2099 mL | 0.4199 mL | 0.5248 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Aloinoside A
Catalog No.:BCX0853
CAS No.:56645-88-6
- Tomentosin
Catalog No.:BCX0852
CAS No.:33649-15-9
- Aloinoside B
Catalog No.:BCX0851
CAS No.:11006-91-0
- Gymnoside I
Catalog No.:BCX0850
CAS No.:899430-01-4
- Pseudoginsenoside Rg3(E)
Catalog No.:BCX0849
CAS No.:1012886-99-5
- 6,7-Dihydroxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone
Catalog No.:BCX0848
CAS No.:626236-07-5
- Annatto
Catalog No.:BCX0847
CAS No.:1393-63-1
- 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one
Catalog No.:BCX0846
CAS No.:28564-83-2
- Triptonoterpene
Catalog No.:BCX0845
CAS No.:99694-87-8
- 2'-Hydroxylagarotetrol
Catalog No.:BCX0844
CAS No.:135308-83-7
- Arteether
Catalog No.:BCX0843
CAS No.:75887-54-6
- 6-Epiagarotetrol
Catalog No.:BCX0842
CAS No.:2580359-99-3
- Tenacissoside C
Catalog No.:BCX0855
CAS No.:107347-58-0
- Tenacissoside D
Catalog No.:BCX0856
CAS No.:107347-57-9
- Tenacissoside B
Catalog No.:BCX0857
CAS No.:107424-13-5
- Tenacissoside E
Catalog No.:BCX0858
CAS No.:107347-56-8
- Interiorin
Catalog No.:BCX0859
CAS No.:119139-55-8
- 6''-Malonylthymosin I
Catalog No.:BCX0860
CAS No.:528606-92-0
- Dieugenol
Catalog No.:BCX0861
CAS No.:4433-08-3
- (+)-5-methoxydeydrodiisoeugenol
Catalog No.:BCX0862
CAS No.:1967042-42-7
- Eucomoside B
Catalog No.:BCX0863
CAS No.:951672-66-5
- Polyporusterone C
Catalog No.:BCX0864
CAS No.:141360-90-9
- Crocin E
Catalog No.:BCX0865
CAS No.:58050-17-2
- Acetyl Dopamine DimerIV
Catalog No.:BCX0866
CAS No.:1632326-65-8
Anti-inflammatory/anti-oxidant properties and the UPLC-QTOF/MS-based metabolomics discrimination of three yellow camellia species.[Pubmed:36076438]
Food Res Int. 2022 Oct;160:111628.
The species of Camellia nitidissima Chi (CC) and C. euphlebia Merr. ex Sealy (CE) are two most important plant sources for commercialized herbal tea (Jinhuacha) worldwide. However, some other species of camellia genus are also sold as alternatives in market due to the great commercial value. In this study, the similarity and difference of CC and CE as well as C.insularis (CI) are comprehensively compared both in chemistry and pharmacology. Based on the ultraperformance liquid chromatography coupled with a hybrid quadrupole orthogonal time-of-flight mass spectrometer(UPLC-QTOF-MS) analysis, a sequential-optimization based new statistical model has been developed by combining the untargeted metabolomics and fingerprint analyses, and successfully applied for chemical pattern recognition and discrimination of three yellow camellias species. The results indicated that CC, CE and CI could be well discriminated with the optimized chemical combination including quercetin-3-O-rhamnoside (C2), Okicamelliaside (C4), Kaempferol 7-O-rhamnoside (C6), Corymboside (C9), asiatic acid-glc-rha-xyl (C11) and 3'-methy-4'-glucoside-ellagic acid (C14). Moreover, the 30 % ethanolic extracts of yellow camellias species presented the optimal activities on anti-inflammation/anti-oxidation in LPS-stimulated Raw264.7 macrophages dose-dependently. The averaged 50 % inhibitory concentrations (IC50) on NO production were 754.68 +/- 50.96, 1182.39 +/- 22.10, 1527.83 +/- 106.24 mug(herb)/mL, and ROS production were 311.70 +/- 26.57, 332.64 +/- 25.46, 917.60 +/- 41.36 mug(herb)/mL for CC, CE and CI, respectively. The results indicated a certain similarity of CC and CE, as well as their significant difference from CI.
Okicamelliaside targets the N-terminal chaperone pocket of HSP90 disrupts the chaperone protein interaction of HSP90-CDC37 and exerts antitumor activity.[Pubmed:34326484]
Acta Pharmacol Sin. 2022 Apr;43(4):1046-1058.
Heat shock protein 90 (HSP90) has been recognized as a crucial target in cancer cells. However, various toxic reactions targeting the ATP binding site of HSP90 may not be the best choice for HSP90 inhibitors. In this paper, an ellagic acid derivative, namely, Okicamelliaside (OCS), with antitumor effects was found. To identify potential anti-cancer mechanisms, an OCS photosensitive probe was applied to target fishing and tracing. Chemical proteomics and protein-drug interaction experiments have shown that HSP90 is a key target for OCS, with a strong binding affinity (K(D) = 6.45 muM). Mutation analysis of the target protein and molecular dynamics simulation revealed that OCS could competitively act on the key Glu-47 site at the N-terminal chaperone pocket of HSP90, where the co-chaperone CDC37 binds to HSP90, affect its stability and reduce the ∆G(bind) of HSP90-CDC37. It was demonstrated that OCS destroys the protein-protein interactions of HSP90-CDC37; selectively affects downstream kinase client proteins of HSP90, including CDK4, P-AKT(473), and P-ERK1/2; and exerts antitumor effects on A549 cells. Furthermore, tumor xenograft experiments demonstrated high antitumor activity and low toxicity of OCS in the same way. Our findings identified a novel N-terminal chaperone pocket natural inhibitor of HSP90, that is, OCS, which selectively inhibits the formation of the HSP90-CDC37 protein complex, and provided further insight into HSP90 inhibitors for anti-cancer candidate drugs.
Anti-Allergic Rhinitis Effects of Medicinal Plants and Their Bioactive Metabolites via Suppression of the Immune System: A Mechanistic Review.[Pubmed:33927634]
Front Pharmacol. 2021 Apr 13;12:660083.
Allergic rhinitis (AR) is a common inflammatory condition of the nasal mucosa and it is an immunoglobulin E-mediated disease. The incidence and prevalence of AR globally have been escalating over recent years. Antihistamines, intranasal corticosteroids, decongestants, intranasal anticholinergics, intranasal cromolyn, leukotriene receptor antagonists and immunotherapy have been used in the treatment of AR. However, there is a need to search for more effective and safer remedies as many of the current treatments have reported side effects. Medicinal plants have been used traditionally to relief symptoms of AR but their efficacy and safety have not been scientifically proven. In this review, up-to-date reports of studies on the anti-allergic rhinitis of several medicinal plants and their bioactive metabolites through suppression of the immune system are compiled and critically analyzed. The plant samples were reported to suppress the productions of immunoglobulin E, cytokines and eosinophils and inhibit histamine release. The suppression of cytokines production was found to be the main mechanistic effect of the plants to give symptomatic relief. The prospect of these medicinal plants as sources of lead molecules for development of therapeutic agents to treat AR is highlighted. Several bioactive metabolites of the plants including shikonin, Okicamelliaside, warifteine, methylwarifteine, luteolin-7-O-rutinoside, tussilagone, petasin, and mangiferin have been identified as potential candidates for development into anti-allergic rhinitis agents. The data collection was mainly from English language articles published in journals, or studies from EBSCOHOST, Medline and Ovid, Scopus, Springer, and Google Scholar databases from the year 1985-2020. The terms or keywords used to find relevant studies were allergic rhinitis OR pollinosis OR hay fever, AND medicinal plant OR single plant OR single herb OR phytotherapy. This comprehensive review serves as a useful resource for medicinal plants with anti-allergic rhinitis potential, understanding the underlying mechanisms of action and for future exploration to find natural product candidates in the development of novel anti-allergic rhinitis agents.
Integrated molecular network and HPLC-UV-FLD analysis to explore antioxidant ingredients in camellia nitidissima Chi.[Pubmed:33733483]
J Food Sci. 2021 Apr;86(4):1296-1305.
At present, screening of active ingredients from natural products for pharmacological and clinical research is mostly time-consuming and costly. In this study, a molecular network (MN) guided high performance liquid chromatography-ultraviolet-fluorescence detector (HPLC-UV-FLD) method was carried out to profile the global antioxidant activity compounds, including the trace amount ingredients in Camellia nitidissima Chi (CNC). Firstly, HPLC-UV-FLD postcolumn derivatization system was utilized to screen the antioxidants. Then the MN of CNC was established via mass spectrometry (MS) data for getting the connection between ingredient structures. As a result, HPLC-UV-FLD indicated three antioxidant ingredients: gallic acid (126.3 mg/g), catechin (564.8 mg/g), and salicylic acid (24.3 mg/g). Combined with the MN, the actives' precise location and connection relationship were clarified based on the structural similarities. A new antioxidant ingredient, Okicamelliaside, was suggested and evaluated at free radical scavenging and enzymatic protection. The novel method of activity and structural correlation analysis based on MN could provide a useful guide for screening trace active ingredients in natural products. PRACTICAL APPLICATION: Three main ingredients were screened out from Camellia nitidissima Chi by HPLC-UV-FLD postcolumn derivatization system. Integrated molecular network and HPLC-UV-FLD analysis, a new type of antioxidant Okicamelliaside was selected. The novel method of activity and structural correlation analysis based on molecular network could provide a useful guide for screening trace active ingredients in natural products.
Synthesis of okicamelliaside, a glucoside of ellagic acid with potent anti-degranulation activity.[Pubmed:23563555]
Biosci Biotechnol Biochem. 2013;77(4):810-3.
Okicamelliaside, a glucoside of ellagic acid with potent anti-degranulation activity, was synthesized from ellagic acid. The regioselectivity, solubility, and high reactivity of the intermediates throughout the synthesis were obtained by the complementary use of triisopropylsilyl (TIPS) and methoxyethoxymethyl (MEM) protective groups on the aglycone skeleton.
Inhibitory effects of an ellagic acid glucoside, okicamelliaside, on antigen-mediated degranulation in rat basophilic leukemia RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice.[Pubmed:22330086]
Int Immunopharmacol. 2012 Apr;12(4):675-81.
Degranulation inhibitors in plants are widely used for prevention and treatment of immediate-type allergy. We previously isolated a new ellagic acid glucoside, Okicamelliaside (OCS), from Camellia japonica leaves for use as a potent degranulation inhibitor. Crude extracts from leaves also suppressed allergic conjunctivitis in rats. In this study, we evaluated the in vivo effect of OCS using a pure sample and performed in vitro experiments to elucidate the mechanism underlying the extraordinary high potency of OCS and its aglycon. The IC(50) values for degranulation of rat basophilic leukemia cells (RBL-2H3) were 14 nM for OCS and 3 muM for aglycon, indicating that the two compounds were approximately 2 to 3 orders of magnitude more potent than the anti-allergic drugs ketotifen fumarate, DSCG, and tranilast (0.17, 3, and >0.3 mM, respectively). Antigen-induced calcium ion (Ca(2+)) elevation was significantly inhibited by OCS and aglycon at all concentrations tested (p<0.05). Upstream of the Ca(2+) elevation in the principle signaling pathway, phosphorylation of Syk (Tyr525/526) and PLCgamma-1 (Tyr783 and Ser1248) were inhibited by OCS and aglycon. In DNA microarray-screening test, OCS inhibited expression of proinflammatory cytokines [interleukin (IL)-4 and IL-13], cytokine-producing signaling factors, and prostaglandin-endoperoxidase 2, indicating that OCS broadly inhibits allergic inflammation. During passive cutaneous anaphylaxis in mice, OCS significantly inhibited vascular hyperpermeability by two administration routes: a single intraperitoneal injection at 10 mg/kg and per os at 5 mg/kg for 7 days (p<0.05). These results suggest the potential for OCS to alleviate symptoms of immediate-type allergy.
Okicamelliaside, an extraordinarily potent anti-degranulation glucoside isolated from leaves of Camellia japonica.[Pubmed:21150097]
Biosci Biotechnol Biochem. 2010;74(12):2532-4.
Guided by anti-degranulation assays, we isolated from leaves of Camellia japonica an ellagic acid glucoside named Okicamelliaside. The structure was elucidated as 3,4-dioxoloellagic acid 4'-O-beta-D-glucopyranoside by spectroscopic and chemical methods. Okicamelliaside was 12,000 times more potent than the antihistaminic drug, ketotifen fumarate, in inhibiting the degranulation of RBL-2H3 cells.


