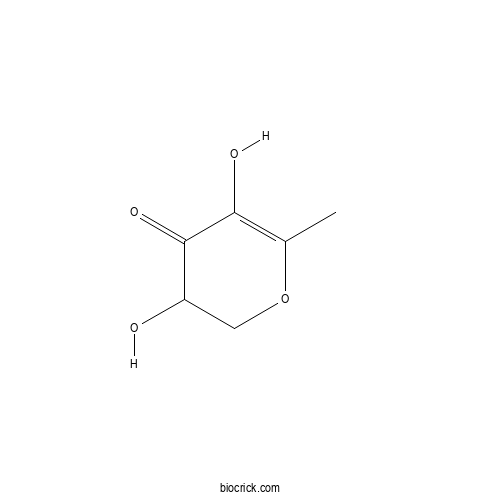
2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-oneCAS# 28564-83-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28564-83-2 | SDF | Download SDF |
| PubChem ID | 119838.0 | Appearance | Powder |
| Formula | C6H8O4 | M.Wt | 144.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,5-dihydroxy-6-methyl-2,3-dihydropyran-4-one | ||
| SMILES | CC1=C(C(=O)C(CO1)O)O | ||
| Standard InChIKey | VOLMSPGWNYJHQQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H8O4/c1-3-5(8)6(9)4(7)2-10-3/h4,7-8H,2H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one Dilution Calculator

2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.9382 mL | 34.6909 mL | 69.3818 mL | 138.7636 mL | 173.4545 mL |
| 5 mM | 1.3876 mL | 6.9382 mL | 13.8764 mL | 27.7527 mL | 34.6909 mL |
| 10 mM | 0.6938 mL | 3.4691 mL | 6.9382 mL | 13.8764 mL | 17.3455 mL |
| 50 mM | 0.1388 mL | 0.6938 mL | 1.3876 mL | 2.7753 mL | 3.4691 mL |
| 100 mM | 0.0694 mL | 0.3469 mL | 0.6938 mL | 1.3876 mL | 1.7345 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Triptonoterpene
Catalog No.:BCX0845
CAS No.:99694-87-8
- 2'-Hydroxylagarotetrol
Catalog No.:BCX0844
CAS No.:135308-83-7
- Arteether
Catalog No.:BCX0843
CAS No.:75887-54-6
- 6-Epiagarotetrol
Catalog No.:BCX0842
CAS No.:2580359-99-3
- (6S,7S,8S)-5,6,7,8-Tetrahydro-6,7,8-trihydroxy-2-(2-phenylethyl)-4H-1-benzopyran-4-one
Catalog No.:BCX0841
CAS No.:2803559-82-0
- Aquilarone C
Catalog No.:BCX0840
CAS No.:1404479-46-4
- 2'-Hydroxylisoagarotetrol
Catalog No.:BCX0839
CAS No.:104926-77-4
- 4H-xanthalongin
Catalog No.:BCX0838
CAS No.:72843-22-2
- 8-epi-helenalin
Catalog No.:BCX0837
CAS No.:97643-91-9
- 4'-Methoxyisoagarotetrol
Catalog No.:BCX0836
CAS No.:104901-10-2
- 6''-O-acetyl-saikosaponin B2
Catalog No.:BCX0835
CAS No.:104121-82-6
- (S)-Goitrin
Catalog No.:BCX0834
CAS No.:500-12-9
- Annatto
Catalog No.:BCX0847
CAS No.:1393-63-1
- 6,7-Dihydroxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone
Catalog No.:BCX0848
CAS No.:626236-07-5
- Pseudoginsenoside Rg3(E)
Catalog No.:BCX0849
CAS No.:1012886-99-5
- Gymnoside I
Catalog No.:BCX0850
CAS No.:899430-01-4
- Aloinoside B
Catalog No.:BCX0851
CAS No.:11006-91-0
- Tomentosin
Catalog No.:BCX0852
CAS No.:33649-15-9
- Aloinoside A
Catalog No.:BCX0853
CAS No.:56645-88-6
- Okicamelliaside
Catalog No.:BCX0854
CAS No.:949148-45-2
- Tenacissoside C
Catalog No.:BCX0855
CAS No.:107347-58-0
- Tenacissoside D
Catalog No.:BCX0856
CAS No.:107347-57-9
- Tenacissoside B
Catalog No.:BCX0857
CAS No.:107424-13-5
- Tenacissoside E
Catalog No.:BCX0858
CAS No.:107347-56-8
Insights into the Regulation Effects of Certain Phenolic Acids on 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one Formation in a Microaqueous Glucose-Proline System.[Pubmed:31339697]
J Agric Food Chem. 2019 Aug 14;67(32):9050-9059.
The control of 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one (DDMP) formation in the Maillard reaction is important to improve the thermally treated food quality as a result of its intense bitterness and potential toxicity. In this work, phenolic acids, such as gallic, protocatechuic, caffeic, and ferulic acids, were applied to modulate DDMP formation in a microaqueous glucose-proline model. The formation of DDMP was inhibited at low concentrations (from 0.1 to 5.0 mM) while enhanced at 10.0 mM gallic, protocatechuic, and caffeic acids. Ferulic acid always inhibited DDMP formation as a result of the absence of catechol groups on its benzene ring. The result indicated that the control of DDMP formation depended upon the concentration and chemical structures of phenolic acids, such as the number of hydroxyl groups. Further studies indicated that the hydroxyl distribution of phenolic acids regulated the peroxide formation in the model reaction system and further changed the development of the oxidation reaction, which affected the degradation of glucose via caramel or Maillard reaction, Amadori rearrangement product oxidation, and 1-deoxyglucosone degradation to form the intermediates.
Comparative analyses of chromatographic fingerprints of the roots of Polygonum multiflorum Thunb. and their processed products using RRLC/DAD/ESI-MS(n).[Pubmed:21674436]
Planta Med. 2011 Nov;77(16):1855-60.
The dried roots of Polygonum multiflorum Thunb. (Heshouwu) and their processed products (Zhi-heshouwu) are widely used in traditional Chinese medicine, yet their therapeutic effects are different. Previous investigations focused mainly on the differences between Heshouwu and Zhi-heshouwu in the contents of several known compounds. In this study, a rapid resolution liquid chromatography-diode array detection/electrospray ionization tandem mass spectrometry (RRLC/DAD/ESI-MS(n)) method was developed for the comparative analysis of the components of Heshouwu and Zhi-heshouwu. A total of 23 compounds were identified or tentatively characterized. We found that 16 batches of Heshouwu and 15 batches of Zhi-heshouwu samples shared eight compounds, including gallic acid; 3,5,4'-tetrahydroxylstilbene-2,3-di-O-glucoside, CIS-2,3,5,4'-tetrahydroxylstilbene-2-O- beta-D-glucoside, trans-2,3,5,4'-tetrahydroxylstilbene-2-O- beta-D-glucoside, emodin-8-O- beta-D-glucoside, physcion-8-O- beta-D-glucoside, emodin, and physcion. Nevertheless, the relative amounts of gallic acid, emodin, and physcion were very high in Zhi-heshouwu samples compared to those in Heshouwu samples. Six compounds disappeared after processing and were unique for Heshouwu: catechin, flavanol gallate dimer, polygonimitin B, emodin-1-O-glucoside, emodin-8-O-(6'-O-malonyl)-glucoside, and physcion-8-O-(6'-O-malonyl)-glucoside. Three compounds were unique for Zhi-heshouwu: hydroxymaltol, 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one, and 5-hydroxymethyl furfural. These results suggest that the types and relative amounts of the chemical components of Heshouwu and Zhi-heshouwu are different.


