TriptonoterpeneCAS# 99694-87-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 99694-87-8 | SDF | Download SDF |
| PubChem ID | 101691231.0 | Appearance | Powder |
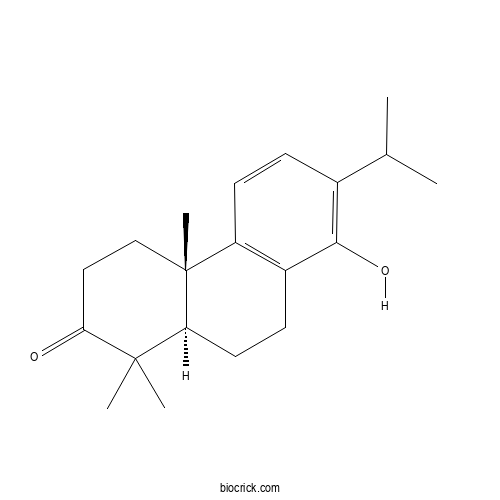
| Formula | C20H28O2 | M.Wt | 300.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4aS,10aR)-8-hydroxy-1,1,4a-trimethyl-7-propan-2-yl-4,9,10,10a-tetrahydro-3H-phenanthren-2-one | ||
| SMILES | CC(C)C1=C(C2=C(C=C1)C3(CCC(=O)C(C3CC2)(C)C)C)O | ||
| Standard InChIKey | WENIWZBFJBCNNG-OXJNMPFZSA-N | ||
| Standard InChI | InChI=1S/C20H28O2/c1-12(2)13-6-8-15-14(18(13)22)7-9-16-19(3,4)17(21)10-11-20(15,16)5/h6,8,12,16,22H,7,9-11H2,1-5H3/t16-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Triptonoterpene Dilution Calculator

Triptonoterpene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3285 mL | 16.6423 mL | 33.2845 mL | 66.569 mL | 83.2113 mL |
| 5 mM | 0.6657 mL | 3.3285 mL | 6.6569 mL | 13.3138 mL | 16.6423 mL |
| 10 mM | 0.3328 mL | 1.6642 mL | 3.3285 mL | 6.6569 mL | 8.3211 mL |
| 50 mM | 0.0666 mL | 0.3328 mL | 0.6657 mL | 1.3314 mL | 1.6642 mL |
| 100 mM | 0.0333 mL | 0.1664 mL | 0.3328 mL | 0.6657 mL | 0.8321 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2'-Hydroxylagarotetrol
Catalog No.:BCX0844
CAS No.:135308-83-7
- Arteether
Catalog No.:BCX0843
CAS No.:75887-54-6
- 6-Epiagarotetrol
Catalog No.:BCX0842
CAS No.:2580359-99-3
- (6S,7S,8S)-5,6,7,8-Tetrahydro-6,7,8-trihydroxy-2-(2-phenylethyl)-4H-1-benzopyran-4-one
Catalog No.:BCX0841
CAS No.:2803559-82-0
- Aquilarone C
Catalog No.:BCX0840
CAS No.:1404479-46-4
- 2'-Hydroxylisoagarotetrol
Catalog No.:BCX0839
CAS No.:104926-77-4
- 4H-xanthalongin
Catalog No.:BCX0838
CAS No.:72843-22-2
- 8-epi-helenalin
Catalog No.:BCX0837
CAS No.:97643-91-9
- 4'-Methoxyisoagarotetrol
Catalog No.:BCX0836
CAS No.:104901-10-2
- 6''-O-acetyl-saikosaponin B2
Catalog No.:BCX0835
CAS No.:104121-82-6
- (S)-Goitrin
Catalog No.:BCX0834
CAS No.:500-12-9
- Aquilarone B
Catalog No.:BCX0833
CAS No.:1404479-45-3
- 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one
Catalog No.:BCX0846
CAS No.:28564-83-2
- Annatto
Catalog No.:BCX0847
CAS No.:1393-63-1
- 6,7-Dihydroxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone
Catalog No.:BCX0848
CAS No.:626236-07-5
- Pseudoginsenoside Rg3(E)
Catalog No.:BCX0849
CAS No.:1012886-99-5
- Gymnoside I
Catalog No.:BCX0850
CAS No.:899430-01-4
- Aloinoside B
Catalog No.:BCX0851
CAS No.:11006-91-0
- Tomentosin
Catalog No.:BCX0852
CAS No.:33649-15-9
- Aloinoside A
Catalog No.:BCX0853
CAS No.:56645-88-6
- Okicamelliaside
Catalog No.:BCX0854
CAS No.:949148-45-2
- Tenacissoside C
Catalog No.:BCX0855
CAS No.:107347-58-0
- Tenacissoside D
Catalog No.:BCX0856
CAS No.:107347-57-9
- Tenacissoside B
Catalog No.:BCX0857
CAS No.:107424-13-5
Triptonoterpene, a Natural Product from Celastrus orbiculatus Thunb, Has Biological Activity against the Metastasis of Gastric Cancer Cells.[Pubmed:36432106]
Molecules. 2022 Nov 18;27(22):8005.
Cancer is one of the greatest threats to human health. Gastric cancer (GC) is the fifth most common malignant tumor in the world. Invasion and metastasis are the major difficulties in the treatment of GC. Herbal medicines and their extracts have a lengthy history of being used to treat tumors in China. The anti-tumoral effects of the natural products derived from herbs have received a great deal of attention. Our previous studies have shown that the traditional Chinese herb Celastrus orbiculatus Thunb extract (COE) can inhibit the invasion and metastasis of GC cells, but the specific anti-cancer components of COE are still unclear. Dozens of natural products from COE have been isolated and identified by HPLC spectroscopy in our previous experiments. Triptonoterpene is one of the active ingredients in COE. In this study, we focused on revealing whether Triptonoterpene has an excellent anti-GC effect and can be used as an effective component of Celastrus orbiculatus Thunb in the treatment of tumors. We first observed that Triptonoterpene reduces GC cell proliferation through CCK-8 assays and colony formation experiments. The cell adhesion assays have shown that Triptonoterpene inhibits adhesion between cells and the cell matrix during tumor invasion. In addition, the cell migration assay has shown that Triptonoterpene inhibits the invasion and migration of GC cells. The high-connotation cell dynamic tracking experiment has also shown the same results. The effects of Triptonoterpene on epidermal mesenchymal transition (EMT)-related and matrix metalloproteinases (MMPs)-related proteins in gastric cancer cells were detected by Western blots. We found that Triptonoterpene could significantly inhibit the changes in EMT-related and invasion and metastasis-related proteins. Altogether, these results suggest that Triptonoterpene is capable of inhibiting the migration and invasion of GC cells. Triptonoterpene, as a natural product from Celastrus orbiculatus Thunb, has significant anti-gastric cancer effects, and is likely to be one of the major equivalent components of Celastrus orbiculatus Thunb.
A new demethyl abietane diterpenoid from the roots of Tripterygium wilfordii.[Pubmed:31230492]
Nat Prod Res. 2020 Nov;34(21):3094-3100.
A new demethyl abietane diterpenoid, Triptotin K (3) together with three known compounds, friedelin (1), canophyllal (2), and Triptonoterpene (4) were isolated from the roots of Tripterygium wilfordii Hook. f. by silica gel column and preparative high performance liquid chromatography. Their structures were determined by extensive NMR data and mass spectroscopic analysis. Triptotin K showed cytotoxic activities against KB, KBv200, HepG2, and MCF-7/ADM cells lines with IC(50) values of 29.88, 36.50, 39.55, and 41.38 muM, respectively.
Characterization of eight terpenoids from tissue cultures of the Chinese herbal plant, Tripterygium wilfordii, by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry.[Pubmed:25237706]
Biomed Chromatogr. 2014 Sep;28(9):1183-92.
In this study, a reliable method for analysis and identification of eight terpenoids in tissue cultures of Tripterygium wilfordii has been established using high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (HPLC-ESI-MS). Our study indicated that sterile seedlings, callus cultures and cell-suspension cultures can rapidly increase the amount of biological materials. HPLC-ESI-MS was used to identify terpenoids from the extracts of these tissue cultures. Triptolide, triptophenolide, celastrol and wilforlide A were unambiguously determined by comparing the retention times, UV spectral data, and mass fragmentation behaviors with those of the reference compounds. Another four compounds were tentatively identified as triptonoterpenol, Triptonoterpene, 22beta-hydroxy-3-oxoolean-12-en-29-oic acid and wilforlide B, based on their UV and mass spectrometry spectra. The quantitative analysis showed that all three materials contain triptolide, triptophenolide, celastrol, wilforlide A, and the contents of the four compounds in the cell-suspension cultures were 53.1, 240, 129 and 964 microg/g, respectively, which were at least 2.0-fold higher than these in the sterile seedlings and callus cultures. Considering the known pharmacological activity of triptolide and celastrol, we recommend the cell-suspension cultures as biological materials for future studies, such as clinical and toxicological studies. The developed method was validated by the evaluation of its precision, linearity, detection limits and recovery, and it was successfully used to identify and quantify the terpenoids in the tissue cultures.
[Chemical constituents from root bark of Tripterygium hypoglaucum].[Pubmed:22256754]
Zhongguo Zhong Yao Za Zhi. 2011 Sep;36(18):2503-6.
OBJECTIVE: To investigate chemical constituents of the root bark of Tripterygium hypoglaucum. METHOD: Compounds were isolated by column chromatography on silica gel and Sephadex LH-20, and their structures were identified on the basis of spectral data (MS, 1H-NMR and 13C-NMR). RESULT: Twelve compounds were isolated and identified as friedelin (1), 3-oxo-olean-9(11),12-diene (2), canophyllal (3), 3-acetoxy oleanolic acid (4), triptophenolide (5), Triptonoterpene methyl ether (6), tricosanoic acid (7), beta-sitosterol (8), stearic acid (9), glut-5-en-3beta,28-diol (10), palmitic acid (11) and daucostorol (12). CONCLUSION: Compounds 1, 2, 3, 7 and 10 were isolated from T. hypoglaucum and 7 from the genus Tripterygium for the first time.
Flow rate gradient high-speed counter-current chromatography separation of five diterpenoids from Triperygium wilfordii and scale-up.[Pubmed:18554602]
J Chromatogr A. 2008 Jul 25;1200(2):129-35.
In this paper, high-speed counter-current chromatography (HSCCC) instruments with different gravitational forces were applied for the separation of bioactive compounds from Triperygium wilfordii Hook.f. The critical parameters including sample concentration, sample volume and flow rate were first optimized on an analytical Mini-DE HSCCC system, and then scaled up to a preparative TBE 300A HSCCC system. Although this scale-up process was performed using different CCC instruments with different centrifuges and gravitational forces, the same resolutions were obtained and the elution time could be predictable. Five diterpenoid compounds and one unknown compound were separated from Triperygium wilfordii Hook.f. by HSCCC with a two-phase solvent system composed of n-hexane-ethyl acetate-methanol-water (HEMW) (3:2:3:2, v/v/v/v). This one-step flow gradient separation produced triptonide (25 mg), isoneotriptophenolide (77 mg), hypolide (83 mg), unknown compound (1 mg), triptophenolide (42 mg), Triptonoterpene methyl ether VI (37 mg) from 320 mg crude extract with purities of 98.2%, 96.6%, 98.1%, 95.3%, 95.1%, and 96.5%, respectively. Their purities and structures were identified by high-performance liquid chromatography, mass spectrometry and NMR. This paper demonstrates that analytical CCC plays an important role in optimizing parameters and scale-up process when analytical CCC and preparative CCC are supplied by different manufacturers with different gravitational forces, and the scale-up process from analytical CCC to preparative CCC is still predictable.
Studies on New Components and Stereochemistry of Diterpenoids from Trypterygium wilfordii.[Pubmed:17265278]
Planta Med. 1988 Aug;54(4):330-2.
An extensive investigation of the petroleum ether extract of TRIPTERYGIUM WILFORDII plants have revealed the presence of four new compounds, Triptonoterpene, neoTriptonoterpene, triptonodiol, and neotriptonolide, in addition to the diterpenes isolated and characterized earlier. The structures of these novel natural products have been elucidated.


