ArteetherCAS# 75887-54-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 75887-54-6 | SDF | Download SDF |
| PubChem ID | 3000469.0 | Appearance | Powder |
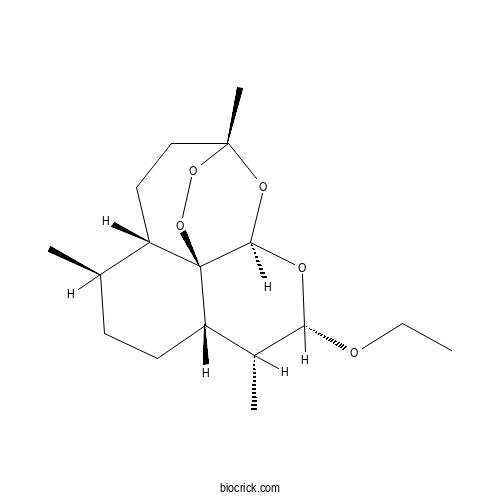
| Formula | C17H28O5 | M.Wt | 312.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,4S,5R,8S,9R,10S,12R,13R)-10-ethoxy-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecane | ||
| SMILES | CCOC1C(C2CCC(C3C24C(O1)OC(CC3)(OO4)C)C)C | ||
| Standard InChIKey | NLYNIRQVMRLPIQ-XQLAAWPRSA-N | ||
| Standard InChI | InChI=1S/C17H28O5/c1-5-18-14-11(3)13-7-6-10(2)12-8-9-16(4)20-15(19-14)17(12,13)22-21-16/h10-15H,5-9H2,1-4H3/t10-,11-,12+,13+,14+,15-,16-,17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Arteether Dilution Calculator

Arteether Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.201 mL | 16.0051 mL | 32.0102 mL | 64.0205 mL | 80.0256 mL |
| 5 mM | 0.6402 mL | 3.201 mL | 6.402 mL | 12.8041 mL | 16.0051 mL |
| 10 mM | 0.3201 mL | 1.6005 mL | 3.201 mL | 6.402 mL | 8.0026 mL |
| 50 mM | 0.064 mL | 0.3201 mL | 0.6402 mL | 1.2804 mL | 1.6005 mL |
| 100 mM | 0.032 mL | 0.1601 mL | 0.3201 mL | 0.6402 mL | 0.8003 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Epiagarotetrol
Catalog No.:BCX0842
CAS No.:2580359-99-3
- (6S,7S,8S)-5,6,7,8-Tetrahydro-6,7,8-trihydroxy-2-(2-phenylethyl)-4H-1-benzopyran-4-one
Catalog No.:BCX0841
CAS No.:2803559-82-0
- Aquilarone C
Catalog No.:BCX0840
CAS No.:1404479-46-4
- 2'-Hydroxylisoagarotetrol
Catalog No.:BCX0839
CAS No.:104926-77-4
- 4H-xanthalongin
Catalog No.:BCX0838
CAS No.:72843-22-2
- 8-epi-helenalin
Catalog No.:BCX0837
CAS No.:97643-91-9
- 4'-Methoxyisoagarotetrol
Catalog No.:BCX0836
CAS No.:104901-10-2
- 6''-O-acetyl-saikosaponin B2
Catalog No.:BCX0835
CAS No.:104121-82-6
- (S)-Goitrin
Catalog No.:BCX0834
CAS No.:500-12-9
- Aquilarone B
Catalog No.:BCX0833
CAS No.:1404479-45-3
- 6''-O-Acetyldaidzin
Catalog No.:BCX0832
CAS No.:71385-83-6
- Artemisitene
Catalog No.:BCX0831
CAS No.:101020-89-7
- 2'-Hydroxylagarotetrol
Catalog No.:BCX0844
CAS No.:135308-83-7
- Triptonoterpene
Catalog No.:BCX0845
CAS No.:99694-87-8
- 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one
Catalog No.:BCX0846
CAS No.:28564-83-2
- Annatto
Catalog No.:BCX0847
CAS No.:1393-63-1
- 6,7-Dihydroxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone
Catalog No.:BCX0848
CAS No.:626236-07-5
- Pseudoginsenoside Rg3(E)
Catalog No.:BCX0849
CAS No.:1012886-99-5
- Gymnoside I
Catalog No.:BCX0850
CAS No.:899430-01-4
- Aloinoside B
Catalog No.:BCX0851
CAS No.:11006-91-0
- Tomentosin
Catalog No.:BCX0852
CAS No.:33649-15-9
- Aloinoside A
Catalog No.:BCX0853
CAS No.:56645-88-6
- Okicamelliaside
Catalog No.:BCX0854
CAS No.:949148-45-2
- Tenacissoside C
Catalog No.:BCX0855
CAS No.:107347-58-0
Role of angiotensin pathway and its target therapy to rescue from experimental cerebral malaria.[Pubmed:38570086]
Microbes Infect. 2024 Apr 2:105333.
Cerebral malaria (CM) induced by Plasmodium falciparum is a devastating neurological complication that may lead the patient to coma and death. This study aimed to protect Plasmodium-infected C57BL6 mice from CM by targeting the angiotensin II type 1 (AT1) receptor, which is considered the common connecting link between hypertension and CM. In CM, AT-1 mediates blood-brain barrier (BBB) damage through the overexpression of beta-catenin. The AT-1-inhibiting drugs, such as irbesartan and losartan, were evaluated for the prevention of CM. The effectiveness of these drugs was determined by the down regulation of beta-catenin, TCF, LEF, ICAM-1, and VCAM-1 in the drug-treated groups. The expression levels of VE-cadherin and vinculin, essential for the maintenance of BBB integrity, were found to be restored in the drug-treated groups. The pro-inflammatory cytokine levels were decreased, and the anti-inflammatory cytokine levels increased with the treatment. As a major highlight, the mean survival time of treated mice was found to be increased even in the absence of treatment with an anti-malarial agent. The combination of irbesartan or losartan with the anti-malarial agent alpha/beta-Arteether has contributed to an 80% cure rate, which is higher than the 60% cure rate observed with alpha/beta-Arteether alone treatment.
Novel ether derivatives of 11-azaartemisinins with high order antimalarial activity against multidrug-resistant Plasmodium yoelii in Swiss mice.[Pubmed:38479483]
Bioorg Med Chem Lett. 2024 May 1;103:129700.
This study investigates cutting-edge synthetic chemistry approaches for designing and producing innovative antimalarial drugs with improved efficacy and fewer adverse effects. Novel amino (-NH(2)) and hydroxy (-OH) functionalized 11-azaartemisinins 9, 12, and 14 were synthesized along with their derivatives 11a, 13a-e, and 15a-b through ART and were tested for their AMA (antimalarial activity) against Plasmodium yoelii via intramuscular (i.m.) and oral routes in Swiss mice. Ether derivative 13c was the most active compound by i.m. route, it has shown 100 % protection at the dose of 12 mg/kg x 4 days and showed 100 % clearance of parasitaemia on day 4 at dose of 6 mg/kg. Amine 11a, ether derivatives 13d, 13e and ether 15a also showed promising antimalarial activity. beta-Arteether gave 100 % protection at the dose of 48 mg/kg x 4 days and 20 % protection at 24 mg/kg x 4 days dose by oral route, while it showed 100 % protection at 6 mg/kg x 4 days and no protection at 3 mg/kg x 4 days by i.m. route.
Comparison of Two Grafted Copolymers, Soluplus and Kollicoat IR, as Solid Dispersion Carriers of Arteether for Oral Delivery Prepared by Different Solvent-Based Methods.[Pubmed:38075813]
ACS Omega. 2023 Nov 27;8(48):45337-45347.
Arteether (ART), an antimalarial drug, belongs to BCS class II and has very low oral bioavailability. Clinically, it is given as a solution in oil by the intramuscular route. Solid dispersion in Soluplus or Kollicoat IR, two commonly used grafted copolymers, may improve its in vitro dissolution and oral bioavailability. ART solid dispersion was prepared by three solvent-based methods: rotary evaporation (ethanol as solvent), spray drying (hydro-alcoholic solvent), and freeze-drying (aqueous solvent). ART-polymer miscibility increases with increasing polymeric concentrations up to 4% or 6%. Spray drying resulted in the highest increment of ART saturation solubility (476.01 +/- 10.01 mg/L) than that of rotary evaporation (432.22 +/- 15.76 mg/L) or freeze-drying (122.97 +/- 2.94 mg/L) in the drug-Soluplus (1:1 w/w) ratio. Also, with Kollicoat IR-based solid dispersion, the same trend was observed. The drug-polymer ratio of 1:3 (w/w) showed a decrease in saturation solubility. Spray-dried products were better for flow properties (Carr index: 21.27 +/- 0.98 for the 1:1 ratio of drug-Soluplus solid dispersion) than the other two methods. An enteric-coated capsule was prepared with an ART-Soluplus (1:1) ratio, selected based on the saturation solubility and downstream feasibility compared with those of Kollicoat IR. Eudragit L-100-coated enteric capsules containing 100 mg equivalent ART showed 88.88 +/- 2.9% drug release in phosphate buffer pH 6.8 medium, which is significantly higher than that in raw drug (<10%) and a physical mixture of the exact composition of solid dispersion (44%). The study concluded that Soluplus possesses better properties as a solid dispersion carrier than those of Kollicoat IR. A stable, partially amorphous solid dispersion of ART was developed that can provide improved oral bioavailability.
Artemisinins inhibit oral candidiasis caused by Candida albicans through the repression on its hyphal development.[Pubmed:37699886]
Int J Oral Sci. 2023 Sep 12;15(1):40.
Candida albicans is the most abundant fungal species in oral cavity. As a smart opportunistic pathogen, it increases the virulence by switching its forms from yeasts to hyphae and becomes the major pathogenic agent for oral candidiasis. However, the overuse of current clinical antifungals and lack of new types of drugs highlight the challenges in the antifungal treatments because of the drug resistance and side effects. Anti-virulence strategy is proved as a practical way to develop new types of anti-infective drugs. Here, seven artemisinins, including artemisinin, dihydroartemisinin, artemisinic acid, dihydroartemisinic acid, artesunate, artemether and Arteether, were employed to target at the hyphal development, the most important virulence factor of C. albicans. Artemisinins failed to affect the growth, but significantly inhibited the hyphal development of C. albicans, including the clinical azole resistant isolates, and reduced their damage to oral epithelial cells, while Arteether showed the strongest activities. The transcriptome suggested that Arteether could affect the energy metabolism of C. albicans. Seven artemisinins were then proved to significantly inhibit the productions of ATP and cAMP, while reduced the hyphal inhibition on RAS1 overexpression strain indicating that artemisinins regulated the Ras1-cAMP-Efg1 pathway to inhibit the hyphal development. Importantly, Arteether significantly inhibited the fungal burden and infections with no systemic toxicity in the murine oropharyngeal candidiasis models in vivo caused by both fluconazole sensitive and resistant strains. Our results for the first time indicated that artemisinins can be potential antifungal compounds against C. albicans infections by targeting at its hyphal development.
[Microbial transformation of artemisinin and its derivatives].[Pubmed:37381950]
Zhongguo Zhong Yao Za Zhi. 2023 Jun;48(11):2876-2895.
Microbial transformation is an efficient enzymatic approach for the structural modification of exogenous compounds to obtain derivatives. Compared with traditional chemical synthesis, the microbial transformation has in fact the undoubtable advantages of strong region-and stereo-selectivity, and a low environmental and economic impact on the production process, which can achieve the reactions challenging to chemical synthesis. Because microbes are equipped with a broad-spectrum of enzymes and therefore can metabolize various substrates, they are not only a significant route for obtaining novel active derivatives, but also an effective tool for mimicking mammal metabolism in vitro. Artemisinin, a sesquiterpene with a peroxy-bridged structure serving as the main active functional group, is a famous antimalarial agent discovered from Artemisia annua L. Some sesquiterpenoids, such as dihydroartemisinin, artemether, and Arteether, have been developed on the basis of artemisinin, which have been successfully marketed and become the first-line antimalarial drugs recommended by WHO. As revealed by pharmacological studies, artemisinin and its derivatives have exhibited extensive biological activities, including antimalarial, antitumor, antiviral, anti-inflammatory, and immunomodulatory. As an efficient approach for structural modification, microbial transformation of artemisinin and its derivatives is an increasingly popular strategy that attracts considerable attention recently, and numerous novel derivatives have been discovered. Herein, this paper reviewed the microbial transformation of artemisinin and its artemisinin, including microbial strains, culture conditions, product isolation and yield, and biological activities, and summarized the advances in microbial transformation in obtaining active derivatives of artemisinin and the simulation of in vivo metabolism of drugs.
Exploring the Potential Mechanism of Artemisinin and Its Derivatives in the Treatment of Osteoporosis Based on Network Pharmacology and Molecular Docking.[Pubmed:36590764]
Comput Math Methods Med. 2022 Dec 22;2022:3976062.
OBJECTIVE: This study is aimed at predicting and contrasting the mechanisms of artemisinin (ARS), dihydroartemisinin (DHA), artesunate (ART), artemether (ARM), and Arteether (ARE) in the treatment of osteoporosis (OP) using network pharmacology and molecular docking. METHODS: The targets of ARS, DHA, ART, ARM, and ARE were obtained from the SwissTargetPrediction. The targets related to OP were obtained from the TTD, DrugBank, Genecards, and DisGeNet databases. Then, the anti-OP targets of ARS, DHA, ART, ARM, and ARE were obtained and compared using the Venn diagram. Afterward, the protein-protein interaction (PPI) networks were built using the STRING database, and Cytoscape was used to select hub targets. Moreover, molecular docking validated the binding association between five molecules and hub targets. Finally, GO enrichment and KEGG pathway enrichment were conducted using the DAVID database. The common pathways of five molecules were analysed. RESULTS: A total of 28, 37, 36, 27, and 33 anti-OP targets of ARS, DHA, ART, ARM, and ARE were acquired. EGFR, EGFR, CASP3, MAPK8, and CASP3 act as the top 1 anti-OP targets of ARS, DHA, ART, ARM, and ARE, respectively. MAPK14 is the common target of five molecules. All five molecules can bind well with these hubs and common targets. Meanwhile, functional annotation showed that MAPK, Serotonergic synapse, AMPK, prolactin, and prolactin signaling pathways are the top 1 anti-OP pathway of ARS, DHA, ART, ARM, and ARE, respectively. IL-17 signaling pathway and prolactin signaling pathway are common anti-OP pathways of five molecules. Besides, GO enrichment showed five biological processes and three molecular functions are common anti-OP mechanisms of five molecules. CONCLUSION: ARS, DHA, ART, ARM and ARE can treat OP through multi-targets and multi pathways, respectively. All five molecules can treat OP by targeting MAPK14 and acting on the IL-17 and prolactin signaling pathways.
Development of Novel Solid Nanostructured Lipid Carriers for Bioavailability Enhancement Using a Quality by Design Approach.[Pubmed:36109467]
AAPS PharmSciTech. 2022 Sep 15;23(7):253.
alpha, beta-Arteether (ART) antimalarial drug is used to treat chloroquine-resistant malaria and cerebral malaria. The drug's solubility in water is relatively low (17 mug/mL), and 40% of the drug degrades in the stomach, resulting in poor bioavailability. This article discusses the quality by design technique used for formulation development and optimization of nanostructured lipid carriers (NLCs). The ART-NLCs were made by solvent diffusion method. To develop solid NLCs, the NLCs were freeze-dried and encapsulated in enteric-coated capsule shells. The prepared NLCs showed particle size ranging between 156.8 +/- 12 nm while zeta potential ranging between - 26.1 +/- 0.22 mV. They also showed high encapsulation efficiency (> 85%) and an amorphous drug's lipid matrix state. Pharmacokinetic parameters of optimized formulation enhance oral bioavailability to 18.45%. These investigations demonstrated the superiority of NLCs for improvement of solubility as well as oral bioavailability of poorly water-soluble drugs.
Comparative efficacy and safety of the artemisinin derivatives compared to quinine for treating severe malaria in children and adults: A systematic update of literature and network meta-analysis.[Pubmed:35857773]
PLoS One. 2022 Jul 20;17(7):e0269391.
BACKGROUND: The artemisinin derivatives are the preferred antimalaria drugs for treating severe Plasmodium falciparum malaria. However, their clinical effectiveness compared to each other is unknown. Our objective, therefore, was to evaluate the efficacy and safety of the artemisinin derivatives and quinine for treating severe P. falciparum malaria in children and adults using a network meta-analysis. METHODS AND FINDINGS: Review protocol was registered with PROSPERO, CRD42020218190. We updated the search strategies of three Cochrane systematic reviews which included published and unpublished randomised control trials (RCTs) that have compared specific artemisinin derivatives to quinine in treating severe malaria. Search included CENTRAL, MEDLINE, Embase, LILACS, ISI Web of Science and trial registries up to February 2021. We screened studies, extracted data, assessed risk of bias, and quality of evidence in duplicate. Separate network meta-analyses in the frequentist framework, using a random effects model, with quinine as reference, were conducted for adults and children, and rankings were produced using p-scores to assess mortality, parasite clearance, coma recovery, fever clearance, neurological sequela and adverse events. Searches identified 818 citations, 33 RCTs were eligible. We pooled 7795 children and 3182 adults. The networks involved artesunate, artemether, rectal artemisinin, Arteether and quinine. Compared to quinine, artesunate reduced mortality in children (risk ratio (RR), 0.76; 95%CI [0.65 to 0.89], moderate quality), adults (RR, 0.55; 95%CI [0.40 to 0.75], moderate quality) and in cerebral malaria (RR, 0.72; 95%CI [0.55 to 0.94], moderate quality). Compared to rectal artemisinin and intramuscular Arteether, the efficacy and safety of parenteral artesunate, and intramuscular artemether in treating severe malaria are not clear. Rankings showed that none of the artemisinin drugs were consistently superior in all the outcomes assessed. Indirect evidence produced were of very low ratings due to suspected publication bias and imprecision. CONCLUSIONS: Artesunate reduces mortality compared to quinine for both adults and children in Asia and Africa including cerebral malaria. The artemisinin derivatives remain the best treatment for severe malaria but their comparative clinical effectiveness is yet to be fully explored.
Promising New Antimalarial Combination Drugs: Garlic and Arteether in Pregnant Mice Infected with Plasmodium berghei.[Pubmed:35016599]
Infect Disord Drug Targets. 2022;22(4):e100122200124.
BACKGROUND: Antimalarial prescription remains a challenge in pregnant women because of maternal and fetal complications. Recently, garlic and alpha-beta-Arteether combination treatment in malariainfected mice conferred protection. The purpose of this study is to evaluate the efficacy of these drugs during malaria in pregnancy and its safety measures. OBJECTIVE: The study evaluates the efficacy of Arteether and garlic combination drugs in protection against malaria-infected pregnant mice. METHODS: Plasmodium berghei-infected pregnant mouse model was used to assess the combination drug efficacy and the outcome of abnormalities of the disease after drug treatment. After optimizing the dose and gestation period, maternal protection was confirmed by parasite clearance in smear and mortality observation. In addition, maternal hematological parameters, different organ histopathology, and IgG levels were documented along with the fetal and infant outcomes. RESULTS: Arteether monotherapy resulted in spontaneous fetal abortion or resorption, while dosage optimization and garlic combination resulted in pregnancy completion and malaria protection. The derangements observed in the histoarchitecture of organs and hematological parameters caused by malaria infection revealed improvement after drug treatment, and the smear observation confirms the clearance of malaria parasite in the peripheral blood, but IgG level was maintained at the same higher level as in malaria-infected mice. CONCLUSION: The first report of an Arteether and garlic combination demonstrating high efficacy in protecting against malaria-infected pregnant mice establishes its safety as a viable possible treatment for pregnancy-associated malaria.
Reduction of the Double Bond of 6-Arylvinyl-1,2,4-trioxanes Leads to a Remarkable Increase in Their Antimalarial Activity against Multidrug-Resistant Plasmodium yoelii nigeriensis in a Swiss Mice Model.[Pubmed:34805707]
ACS Omega. 2021 Nov 4;6(45):30790-30799.
Novel 6-arylethyl-1,2,4-trioxanes6a-i and 7a-i are easily accessible in one step from the diimide reduction of 6-arylvinyl-1,2,4-trioxanes 5a-i. All of these new trioxanes were assessed for their oral antimalarial activity against multidrug-resistant Plasmodium yoelii nigeriensis in a Swiss mice model. Most of the saturated trioxanes 6c, 6f, 6g, 6h, and 6i, the active compounds of the series, provided 100% protection to the malaria-infected mice at a dose of 24 mg/kg x 4 days. Further, trioxane 6i, the most active compound of the series, also showed 100% protection even at a dose of 12 mg/kg x 4 days and 20% protection at a dose of 6 mg/kg x 4 days. In this model, beta-Arteether provided 100% protection at a dose of 48 mg/kg x 4 days and only 20% protection at a dose of 24 mg/kg x 4 days via the oral route, which was found to exhibit 4-fold antimalarial activity compared with the currently used drug beta-Arteether.
Molecular Docking and Dynamics Simulation Revealed Ivermectin as Potential Drug against Schistosoma-Associated Bladder Cancer Targeting Protein Signaling: Computational Drug Repositioning Approach.[Pubmed:34684095]
Medicina (Kaunas). 2021 Oct 3;57(10):1058.
Urogenital schistosomiasis is caused by Schistosoma haematobium (S. haematobium) infection, which has been linked to the development of bladder cancer. In this study, three repurposing drugs, ivermectin, Arteether and praziquantel, were screened to find the potent drug-repurposing candidate against the Schistosoma-associated bladder cancer (SABC) in humans by using computational methods. The biology of most glutathione S-transferases (GSTs) proteins and vascular endothelial growth factor (VEGF) is complex and multifaceted, according to recent evidence, and these proteins actively participate in many tumorigenic processes such as cell proliferation, cell survival and drug resistance. The VEGF and GSTs are now widely acknowledged as an important target for antitumor therapy. Thus, in this present study, ivermectin displayed promising inhibition of bladder cancer cells via targeting VEGF and GSTs signaling. Moreover, molecular docking and molecular dynamics (MD) simulation analysis revealed that ivermectin efficiently targeted the binding pockets of VEGF receptor proteins and possessed stable dynamics behavior at binding sites. Therefore, we proposed here that these compounds must be tested experimentally against VEGF and GST signaling in order to control SABC. Our study lies within the idea of discovering repurposing drugs as inhibitors against the different types of human cancers by targeting essential pathways in order to accelerate the drug development cycle.
pH-dependent rearrangement determines the iron-activation and antitumor activity of artemisinins.[Pubmed:33359684]
Free Radic Biol Med. 2021 Feb 1;163:234-242.
The action mechanisms of artemisinins remains elusive for decades, and one long-standing question is whether the indispensable peroxide group is activated by iron or heme. Although heme usually reacts faster with artemisinins than iron does, we have found that rearrangement of dihydroartemisinin (DHA) into monoketo-aldehyde-peroxyhemiacetal (MKA) under physiological conditions can significantly enhance its reaction towards iron. The rearrangement is pH-dependent and the derived MKA is identified by LC-MS in the cellular metabolites of DHA in cancer cells. MKA reacts quickly with ferrous irons to afford reactive carbon-centered radicals and can inhibit enzyme activities in vitro. Moreover, MKA oxidizes ferrous irons to ferric irons, which may explain the effect of DHA on decreasing cellular labile iron pool (LIP). Both addition of exogenous iron and increase in LIP via triggering ferroptosis can enhance the cytotoxicity of DHA against cancer cells. While artesunate (ATS) can also decompose to MKA after hydrolyzing into DHA, the other artemisinins of lower antitumor activity, e.g. artemisinin (ART), artemether (ATM) and Arteether (ATE), exhibit negligible hydrolysis and rearrangement under the same conditions. Our study reveals the vital role of molecular rearrangement to the activation and activity of artemisinins and provides a new strategy for designing antitumor molecules containing endoperoxide group.
Individual and combined anti-trypanosomal effects of arteether and diminazene aceturate in the treatment of experimental Trypanosoma brucei brucei infection in rats.[Pubmed:33132597]
Vet World. 2020 Sep;13(9):1858-1862.
AIM: Trypanosomosis is a vital protozoan disease of man and animals with devastating consequences in the tropical parts of the world, necessitating the investigation of the effects of diminazene aceturate (DA) and Arteether (AR) on Trypanosoma brucei brucei experimental infection in rats. MATERIALS AND METHODS: We used a total of 98 rats, which were divided into 14 groups (A-N) of seven rats each over 36 days after acclimatizing them. We administered 1x10(6) trypanosomes to the infected groups (B-N) with Group A as the unexposed control rats. Groups C-F became the infected and treated rats with 3.5 mg/kg, 7.0 mg/kg, 10.5 mg/kg, and 14.0 mg/kg of DA while Groups G-J became the infected and treated rats with 0.01 ml/kg, 0.02 ml/kg, 0.03 ml/kg, and 0.04 ml/kg of AR. Groups K-N became infected and treated rats with DA and AR combinations at similar doses. RESULTS: Parasitemia suppression occurred in Groups G-J only but became cleared in Groups C-F and K-N. Survival time varied significantly (p<0.05) between Group B and the other infected groups. We recorded anemia in all the infected rats while significant (p<0.05) splenomegaly and hepatomegaly occurred in Groups G-J only compared to the other groups. CONCLUSION: AR did not inhibit or potentiate the anti-trypanosomal efficacy of DA, and therefore, it is comparatively less effective in combating T. brucei infection at the present doses and treatment regimen.
Inhibition of prostate cancer cell line (PC-3) by anhydrodihydroartemisinin (ADHA) through caspase-dependent pathway.[Pubmed:32483407]
EXCLI J. 2020 May 11;19:613-619.
Cancer is a generic term for a large group of diseases characterized by the growth of abnormal cells, which is the second leading cause of death globally. To treat cancer, currently, a number of anticancer drugs belonging to various classes chemically are available. The discovery of artemisinin and its derivatives such as artesunate, Arteether, and artemether became a milestone in the cure for malaria. Here, we report the anti-cancer property of anhydrodihydroartemisinin (ADHA) - a semisynthetic derivative of artemisinin against prostate cancer cell line PC-3. ADHA was found to be inhibiting growth of PC-3 cells. ADHA was also found to be inhibiting migration of PC-3 cells. At molecular level, ADHA was found to be inhibiting the expression of c-Jun, p-c-Jun, p-Akt and NF-kappaB and activated caspase 3 and 7. The results show that ADHA like few other artemisinin derivatives hold potential to be used as an anti-cancer agent against prostate cancer cells.


