ArtemisiteneCAS# 101020-89-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 101020-89-7 | SDF | Download SDF |
| PubChem ID | 154701586.0 | Appearance | Powder |
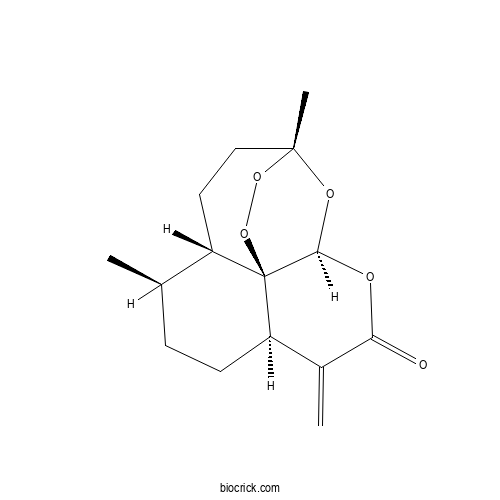
| Formula | C15H20O5 | M.Wt | 280.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,4S,5R,8R,12S,13R)-1,5-dimethyl-9-methylidene-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecan-10-one | ||
| SMILES | CC1CCC2C(=C)C(=O)OC3C24C1CCC(O3)(OO4)C | ||
| Standard InChIKey | IGEBZMMCKFUABB-WJYBAWFFSA-N | ||
| Standard InChI | InChI=1S/C15H20O5/c1-8-4-5-11-9(2)12(16)17-13-15(11)10(8)6-7-14(3,18-13)19-20-15/h8,10-11,13H,2,4-7H2,1,3H3/t8-,10+,11-,13-,14-,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Artemisitene Dilution Calculator

Artemisitene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5674 mL | 17.8368 mL | 35.6735 mL | 71.347 mL | 89.1838 mL |
| 5 mM | 0.7135 mL | 3.5674 mL | 7.1347 mL | 14.2694 mL | 17.8368 mL |
| 10 mM | 0.3567 mL | 1.7837 mL | 3.5674 mL | 7.1347 mL | 8.9184 mL |
| 50 mM | 0.0713 mL | 0.3567 mL | 0.7135 mL | 1.4269 mL | 1.7837 mL |
| 100 mM | 0.0357 mL | 0.1784 mL | 0.3567 mL | 0.7135 mL | 0.8918 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Paeonoside
Catalog No.:BCX0830
CAS No.:20309-70-0
- 4′′,5′′-dehydroisopsoralidin
Catalog No.:BCX0829
CAS No.:65639-51-2
- Quercetin 3-O-β-D-Glucuronide 6''-Methyl Ester
Catalog No.:BCX0828
CAS No.:79543-28-5
- Platycoside F
Catalog No.:BCX0827
CAS No.:314756-03-1
- 6',6''- dimethyl glycyrrhizinate
Catalog No.:BCX0826
CAS No.:114006-81-4
- Heptasaccharide
Catalog No.:BCX0825
CAS No.:121591-98-8
- Licorice glycoside C2
Catalog No.:BCX0824
CAS No.:202657-55-4
- Picfeltarraegenin I
Catalog No.:BCX0823
CAS No.:82145-63-9
- 6''- methyl glycyrrhizinate
Catalog No.:BCX0822
CAS No.:1186016-30-7
- Tribuloside
Catalog No.:BCX0821
CAS No.:22153-44-2
- (25R)-26-O-β-D-Glucopyranosyl-22-hydroxy-5β-furost-3β,26-diol-3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside
Catalog No.:BCX0820
CAS No.:897386-27-5
- Coreoside B
Catalog No.:BCX0819
CAS No.:1580464-83-0
- 6''-O-Acetyldaidzin
Catalog No.:BCX0832
CAS No.:71385-83-6
- Aquilarone B
Catalog No.:BCX0833
CAS No.:1404479-45-3
- (S)-Goitrin
Catalog No.:BCX0834
CAS No.:500-12-9
- 6''-O-acetyl-saikosaponin B2
Catalog No.:BCX0835
CAS No.:104121-82-6
- 4'-Methoxyisoagarotetrol
Catalog No.:BCX0836
CAS No.:104901-10-2
- 8-epi-helenalin
Catalog No.:BCX0837
CAS No.:97643-91-9
- 4H-xanthalongin
Catalog No.:BCX0838
CAS No.:72843-22-2
- 2'-Hydroxylisoagarotetrol
Catalog No.:BCX0839
CAS No.:104926-77-4
- Aquilarone C
Catalog No.:BCX0840
CAS No.:1404479-46-4
- (6S,7S,8S)-5,6,7,8-Tetrahydro-6,7,8-trihydroxy-2-(2-phenylethyl)-4H-1-benzopyran-4-one
Catalog No.:BCX0841
CAS No.:2803559-82-0
- 6-Epiagarotetrol
Catalog No.:BCX0842
CAS No.:2580359-99-3
- Arteether
Catalog No.:BCX0843
CAS No.:75887-54-6
[Simultaneous determination of seven artemisinin-related compounds in Artemisia annua by UPLC-QQQ-MS/MS].[Pubmed:38621973]
Zhongguo Zhong Yao Za Zhi. 2024 Mar;49(5):1260-1265.
A variety of compounds in Artemisia annua were simultaneously determined to evaluate the quality of A. annua from multiple perspectives. A method based on ultra-high performance liquid chromatography-triple quadrupole tandem mass spectrometry(UPLC-QQQ-MS/MS) was established for the simultaneous determination of seven compounds: amorpha-4,11-diene, artemisinic aldehyde, dihydroartemisinic acid, artemisinic acid, artemisinin B, Artemisitene, and artemisinin, in A. annua. The content of the seven compounds in different tissues(roots, stems, leaves, and lateral branches) of A. annua were compared. The roots, stems, leaves, and lateral branches of four-month-old A. annua were collected and the content of seven artemisinin-related compounds in different tissues was determined. A multi-reaction monitoring(MRM) acquisition mode of UPLC-QQQ-MS/MS was used, with a positive ion mode of atmospheric pressure chemical ion source(APCI). Chromatographic separation was achieved on an Eclipse Plus RRHD C_(18) column(2.1 mmx50 mm, 1.8 mum). The gradient elution was performed with the mobile phase consisted of formic acid(0.1%)-ammonium formate(5 mmol.L~(-1))(A) and the methanol(B) gradient program of 0-8 min, 55%-100% B, 8-11 min, 100% B, and equilibrium for 3 min, the flow rate of 0.6 mL.min~(-1), the column temperature of 40 ℃, the injection volume of 5 muL, and the detection time of 8 min. Through methodological investigation, a method based on UPLC-QQQ-MS/MS was established for the simultaneous quantitative determination of seven representative compounds involved in the biosynthesis of artemisinin. The content of artemisinin in A. annua was higher than that of artemisinin B, and the content of artemisinin and dihydroartemisinic acid were high in all the tissues of A. annua. The content of the seven compounds varied considerably in different tissues, with the highest levels in the leaves and neither artemisinene nor artemisinic aldehyde was detected in the roots. In this study, a quantitative method based on UPLC-QQQ-MS/MS for the simultaneous determination of seven representative compounds involved in the biosynthesis of artemisinin was established, which was accurate, sensitive, and highly efficient, and can be used for determining the content of artemisinin-related compounds in A. annua, breeding new varieties, and controlling the quality of Chinese medicinal materials.
Artemisinins: Promising drug candidates for the treatment of autoimmune diseases.[Pubmed:38054758]
Med Res Rev. 2024 Mar;44(2):867-891.
Autoimmune diseases are characterized by the immune system's attack on one's own tissues which are highly diverse and diseases differ in severity, causing damage in virtually all human systems including connective tissue (e.g., rheumatoid arthritis), neurological system (e.g., multiple sclerosis) and digestive system (e.g., inflammatory bowel disease). Historically, treatments normally include pain-killing medication, anti-inflammatory drugs, corticosteroids, and immunosuppressant drugs. However, given the above characteristics, treatment of autoimmune diseases has always been a challenge. Artemisinin is a natural sesquiterpene lactone initially extracted and separated from Chinese medicine Artemisia annua L., which has a long history of curing malaria. Artemisinin's derivatives such as artesunate, dihydroartemisinin, artemether, Artemisitene, and so forth, are a family of artemisinins with antimalarial activity. Over the past decades, accumulating evidence have indicated the promising therapeutic potential of artemisinins in autoimmune diseases. Herein, we systematically summarized the research regarding the immunoregulatory properties of artemisinins including artemisinin and its derivatives, discussing their potential therapeutic viability toward major autoimmune diseases and the underlying mechanisms. This review will provide new directions for basic research and clinical translational medicine of artemisinins.
Medicinal Plants, Phytochemicals and Regulation of the NLRP3 Inflammasome in Inflammatory Bowel Diseases: A Comprehensive Review.[Pubmed:37367886]
Metabolites. 2023 Jun 6;13(6):728.
Ongoing research explores the underlying causes of ulcerative colitis and Crohn's disease. Many experts suggest that dysbiosis in the gut microbiota and genetic, immunological, and environmental factors play significant roles. The term "microbiota" pertains to the collective community of microorganisms, including bacteria, viruses, and fungi, that reside within the gastrointestinal tract, with a particular emphasis on the colon. When there is an imbalance or disruption in the composition of the gut microbiota, it is referred to as dysbiosis. Dysbiosis can trigger inflammation in the intestinal cells and disrupt the innate immune system, leading to oxidative stress, redox signaling, electrophilic stress, and inflammation. The Nod-like Receptor (NLR) Family Pyrin Domain Containing 3 (NLRP3) inflammasome, a key regulator found in immunological and epithelial cells, is crucial in inducing inflammatory diseases, promoting immune responses to the gut microbiota, and regulating the integrity of the intestinal epithelium. Its downstream effectors include caspase-1 and interleukin (IL)-1beta. The present study investigated the therapeutic potential of 13 medicinal plants, such as Litsea cubeba, Artemisia anomala, Piper nigrum, Morus macroura, and Agrimonia pilosa, and 29 phytocompounds such as Artemisitene, morroniside, protopine, ferulic acid, quercetin, picroside II, and hydroxytyrosol on in vitro and in vivo models of inflammatory bowel diseases (IBD), with a focus on their effects on the NLRP3 inflammasome. The observed effects of these treatments included reductions in IL-1beta, tumor necrosis factor-alpha, IL-6, interferon-gamma, and caspase levels, and increased expression of antioxidant enzymes, IL-4, and IL-10, as well as regulation of gut microbiota. These effects could potentially provide substantial advantages in treating IBD with few or no adverse effects as caused by synthetic anti-inflammatory and immunomodulated drugs. However, additional research is necessary to validate these findings clinically and to develop effective treatments that can benefit individuals who suffer from these diseases.
Artemisitene Alters LPS-Induced Oxidative stress, inflammation and Ferroptosis in Liver Through Nrf2/HO-1 and NF-kB Pathway.[Pubmed:37180725]
Front Pharmacol. 2023 Apr 25;14:1177542.
The liver plays a critical role in sepsis, which is a serious worldwide public health problem. A novel mechanism of controlled cell death called ferroptosis has recently been described. Disrupted redox equilibrium, excessive iron, and enhanced lipid peroxidation are key features of ferroptosis. It is unknown how ferroptosis affects liver damage caused by sepsis. In the present study, we aimed to elucidate the pathways and explore the impact of Artemisitene (ATT) on ferroptosis in sepsis-induced liver injury. Our findings demonstrated that ATT significantly decreased liver damage and ferroptotic characteristics. Additionally, ATT significantly reduced the expression of the nuclear factor-kappaB (NF-kappaB) subunit to reduce LPS-induced hepatic oxidative stress and inflammation and upregulated the expression of nuclear factor-erythroid 2 (NF-E2)-related factor 2 (Nrf2) and its downstream protein heme oxygenase 1 (HO-1). This may offer a new strategy for preventing LPS-induced hepatic injury.
Artemisitene suppresses rheumatoid arthritis progression via modulating METTL3-mediated N6-methyladenosine modification of ICAM2 mRNA in fibroblast-like synoviocytes.[Pubmed:36536495]
Clin Transl Med. 2022 Dec;12(12):e1148.
BACKGROUND: Rheumatoid arthritis (RA) is a chronic autoimmune disease. We previously revealed that the natural compound Artemisitene (ATT) exhibits excellent broad anticancer activities without toxicity on normal tissues. Nevertheless, the effect of ATT on RA is undiscovered. Herein, we aim to study the effect and potential mechanism of ATT on RA management. METHODS: A collagen-induced arthritis (CIA) mouse model was employed to confirm the anti-RA potential of ATT. Cell Counting Kit-8 (CCK-8) and 5-ethynyl-2'-deoxyuridine (EdU) assays, cell cycle and apoptosis analysis, immunofluorescence, migration and invasion assays, quantitative real-time PCR (RT-qPCR), Western blot, RNA-sequencing (RNA-seq) analysis, plasmid construction and lentivirus infection, and methylated RNA immunoprecipitation and chromatin immunoprecipitation assays, were carried out to confirm the effect and potential mechanism of ATT on RA management. RESULTS: ATT relieved CIA in mice. ATT inhibited proliferation and induced apoptosis of RA-fibroblast-like synoviocytes (FLSs). ATT restrained RA-FLSs migration and invasion via suppressing epithelial-mesenchymal transition. RNA-sequencing analysis and bioinformatics analysis identified intercellular adhesion molecule 2 (ICAM2) as a promoter of RA progression in RA-FLSs. ATT inhibits RA progression by suppressing ICAM2/phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/p300 pathway in RA-FLSs. Moreover, ATT inhibited methyltransferase-like 3 (METTL3)-mediated N6-methyladenosine methylation of ICAM2 mRNA in RA-FLSs. Interestingly, p300 directly facilitated METTL3 transcription, which could be restrained by ATT in RA-FLSs. Importantly, METTL3, ICAM2 and p300 expressions in synovium tissues of RA patients were related to clinical characteristics and therapy response. CONCLUSIONS: We provided strong evidence that ATT has therapeutic potential for RA management by suppressing proliferation, migration and invasion, in addition to inducing apoptosis of RA-FLSs through modulating METTL3/ICAM2/PI3K/AKT/p300 feedback loop, supplying the fundamental basis for the clinical application of ATT in RA therapy. Moreover, METTL3, ICAM2 and p300 might serve as biomarkers for the therapy response of RA patients.
Artemisinin-derived artemisitene blocks ROS-mediated NLRP3 inflammasome and alleviates ulcerative colitis.[Pubmed:36384076]
Int Immunopharmacol. 2022 Dec;113(Pt B):109431.
Artemisinins are well-known antimalarial drugs with clinical safety. In addition to antimalarial effects, their anti-inflammatory and immunoregulatory properties have recently attracted much attention in the treatment of inflammatory diseases. However, these artemisinins only have sub-millimolar anti-inflammatory activity in vitro, which may pose a high risk of toxicity in vivo with high doses of artemisinins. Here, we identified another derivative, Artemisitene, which can increase the activity of inhibiting the NLRP3 pathway by more than 200-fold through introducing a covalent binding group while retaining the peroxide bridge structure. Mechanistically, Artemisitene inhibits the production of ROS (especially mtROS) and prevents the assembly and activation of NLRP3 inflammasome, thereby inhibiting IL-1beta production. In addition, it can also block IL-1beta secretion mediated by NLRC4 and AIM2 inflammasome and IL-6 production. Furthermore, treatment with Artemisitene significantly attenuated inflammatory response in DSS-induced ulcerative colitis. Our work provides a potential artemisinin derivative, which is worthy of further structural optimization based on pharmacokinetic properties as a drug candidate for inflammatory disorders.
Keap1 Cystenine 151 as a Potential Target for Artemisitene-Induced Nrf2 Activation.[Pubmed:31737667]
Biomed Res Int. 2019 Oct 15;2019:5198138.
Artemisitene (ATT) activates the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) by increasing its stabilization and reducing ubiquitination. The cysteine (Cys) residues of the cytosolic Nrf2 repressor Kelch-like ECH-associated protein-1 (Keap1) function as redox sensors and may be crucial in activating Nrf2. To determine whether ATT-induced Nrf2 activation is dependent on the modification of Keap1 and to elucidate the underlying mechanism, we transfected cell lines with six different Keap1 mutant constructs, each with a Cys (-77, -151, -257, -273, -288, and -297) to Ser substitution. Only the Cys151Ser mutant prevented ATT-mediated activation of Nrf2, indicating that the Cys151 residue of Keap1 likely interacts with ATT and is essential for Nrf2 stabilization and transcription of downstream genes. Our finding provides a pharmacological basis for using Artemisitene against oxidative stress-related diseases.
Anti-proliferative and anti-malarial activities of spiroisoxazoline analogues of artemisinin.[Pubmed:30537298]
Arch Pharm (Weinheim). 2018 Dec 10:e1800192.
A series of spiroisoxazoline analogues of artemisinin was synthesized by employing 1,3-dipolar cycloaddition between various in situ generated nitrile oxides and Artemisitene. All the synthesized compounds were tested for their anti-proliferative and anti-malarial activities. Among the compounds tested, compound 11a was found to be potent against the HCT-15 cancer cell line with IC(50) = 4.04 muM when compared to 5-fluorouracil (IC(50) = 35.53 muM). DNA cell cycle analysis shows that 11a was inhibiting cell proliferation at the G2/M phase. Compound 11b was found to be most active against Plasmodium falciparum with IC(50) = 0.1 muM and also blocked host hemoglobin hydrolysis by the falcipain-3 receptor. It was demonstrated to have better dynamics of parasite killing efficiency than artemisinin. Molecular docking studies revealed that these compounds interacted with falcipain-3 receptor sites.
Detailed Phytochemical Analysis of High- and Low Artemisinin-Producing Chemotypes of Artemisia annua.[Pubmed:29868094]
Front Plant Sci. 2018 May 18;9:641.
Chemical derivatives of artemisinin, a sesquiterpene lactone produced by Artemisia annua, are the active ingredient in the most effective treatment for malaria. Comprehensive phytochemical analysis of two contrasting chemotypes of A. annua resulted in the characterization of over 80 natural products by NMR, more than 20 of which are novel and described here for the first time. Analysis of high- and low-artemisinin producing (HAP and LAP) chemotypes of A. annua confirmed the latter to have a low level of DBR2 (artemisinic aldehyde Delta(11(13)) reductase) gene expression. Here we show that the LAP chemotype accumulates high levels of artemisinic acid, arteannuin B, epi-deoxyarteannuin B and other amorpha-4,11-diene derived sesquiterpenes which are unsaturated at the 11,13-position. By contrast, the HAP chemotype is rich in sesquiterpenes saturated at the 11,13-position (dihydroartemisinic acid, artemisinin and dihydro-epi-deoxyarteannunin B), which is consistent with higher expression levels of DBR2, and also with the presence of a HAP-chemotype version of CYP71AV1 (amorpha-4,11-diene C-12 oxidase). Our results indicate that the conversion steps from artemisinic acid to arteannuin B, epi-deoxyarteannuin B and Artemisitene in the LAP chemotype are non-enzymatic and parallel the non-enzymatic conversion of DHAA to artemisinin and dihyro-epi-deoxyarteannuin B in the HAP chemotype. Interestingly, artemisinic acid in the LAP chemotype preferentially converts to arteannuin B rather than the endoperoxide bridge containing Artemisitene. In contrast, in the HAP chemotype, DHAA preferentially converts to artemisinin. Broader metabolomic and transcriptomic profiling revealed significantly different terpenoid profiles and related terpenoid gene expression in these two morphologically distinct chemotypes.
Artemisitene suppresses tumorigenesis by inducing DNA damage through deregulating c-Myc-topoisomerase pathway.[Pubmed:29795406]
Oncogene. 2018 Sep;37(37):5079-5087.
Cancer chemotherapeutic agents such as doxorubicin are DNA damage inducers that also kill normal cells, making them highly toxic to cancer patients. To improve the efficacy and safety of chemotherapy, it is important to develop new chemotherapeutic agents that selectively kill cancer cells. Here we demonstrate that Artemisitene (ATT), a natural derivative of the antimalarial drug artemisinin, selectively induces DNA double-stranded breaks (DSBs) and apoptosis in various human cancer cells by suppressing the expression of topoisomerases in human cancer cells. ATT effectively kills human cancer cells without apparent cytotoxicity on normal human cells or mouse liver and kidney. We discovered that c-Myc induces the expression of topoisomerases to prevent accumulation of DNA damage in human cancer cells. ATT selectively destabilizes c-Myc in human cancer cells by promoting the ubiquitination of c-Myc through the specific induction of the c-Myc E3 ligase NEDD4. Therefore, ATT represents a promising new chemotherapeutic drug candidate that can eliminate human cancer cells with minimized cytotoxic effects on normal cells.
Determination of artemisitene in rat plasma by ultra-performance liquid chromatography/tandem mass spectrometry and its application in pharmacokinetics.[Pubmed:28403574]
Rapid Commun Mass Spectrom. 2017 Jul 15;31(13):1121-1128.
RATIONALE: Artemisitene shows a wide variety of pharmacological activities, such as antioxidant protection in vitro and in vivo. It has been identified as a novel Nrf2 inducer. However, there is no report on an ultra-performance liquid chromatography/tandem mass spectrometry (UPLC/MS/MS) method to quantitate Artemisitene in rat plasma and its application to a pharmacokinetic profile study. METHODS: An ACQUITY UPLC BEH Symmetry Shield RP18 column (1.7 mum, 2.1 mm x 100 mm) was used at a flow rate of 0.3 mL.min(-1) . Mass detection was performed by electrospray ionization tandem mass spectrometry via multiple reaction monitoring (MRM) in positive mode. Plasma samples were pre-treated by a single-step extraction with 0.1% formic acid aqueous solutions-acetonitrile, and tolbutamide was used as internal standard. RESULTS: The calibration curve was from 0.98 to 1000 ng∙mL(-1) (r(2) = 0.995). The extraction recoveries were 61.5-79.4% and 81.7-94.6% for Artemisitene and tolbutamide, respectively. The lower limit of quantification (LLOQ) was 0.98 ng∙mL(-1) . The absolute bioavailability of Artemisitene was 3.7% after intravenous and oral administration in rats. CONCLUSIONS: The UPLC/MS/MS assay was validated for linearity, accuracy, stability, extraction recovery, matrix effects, and intra-day and inter-day precision. The method, for the first time, achieved some pharmacokinetic parameters and was successfully applied to a pharmacokinetic study Copyright (c) 2017 John Wiley & Sons, Ltd.
Artemisitene activates the Nrf2-dependent antioxidant response and protects against bleomycin-induced lung injury.[Pubmed:27006451]
FASEB J. 2016 Jul;30(7):2500-10.
The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) is a crucial regulator of the cellular antioxidant response and xenobiotic metabolism. Activation of the Nrf2 signaling pathway has been demonstrated to confer protection against environmental insults and prevent disease or inhibit the progression of diseases related to oxidative stress. In an attempt to identify novel improved Nrf2 inducers for systemic protection against tissue damage by environmental insults, we identified Artemisitene as a novel Nrf2 activator using antioxidant responsive element luciferase assay in MDA-MB-231 cells. Further studies suggest that Artemisitene activates Nrf2 by decreasing Nrf2 ubiquitination and increasing its stability. In Nrf2 wild-type mice, systemic administration of Artemisitene strongly inhibits bleomycin-induced lung damage. Artemisitene represents a novel class of Nrf2 inducer, and Artemisitene-based therapeutic approach targeting Nrf2 may also provide antioxidant protection for humans against tissue damage by toxic chemicals.-Chen, W., Li, S., Li, J., Zhou, W., Wu, S., Xu, S., Cui, K., Zhang, D. D., Liu, B. Artemisitene activates the Nrf2-dependent antioxidant response and protects against bleomycin-induced lung injury.
Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells.[Pubmed:26407947]
Phytomedicine. 2015 Oct 15;22(11):1045-54.
BACKGROUND: Apoptosis and other forms of cell death have been intensively investigated in the past years to explain the mode of action of synthetic anticancer drugs and natural products. Recently, a new form of cell death emerged, which was termed ferroptosis, because it depends on intracellular iron. Here, the role of genes involved in iron metabolism and homeostasis for the cytotoxicity of ten artemisinin derivatives have been systematically investigated. MATERIAL AND METHODS: Log10IC50 values of 10 artemisinin derivatives (artesunate, artemether, arteether, artenimol, Artemisitene, arteanuin B, another monomeric artemisinin derivative and three artemisinin dimer molecules) were correlated to the microarray-based mRNA expression of 30 iron-related genes in 60 cell lines of the National Cancer Institute (NCI, USA) as determined in 218 different microarray hybridization experiments. The effect of desferoxamine and ferrostatin-1 on the cytotoxicity of artenimol of CCRF-CEM cells was determined by resazurin assays. The mRNA expression of TFRC was exemplarily validated by immunohistochemical detection of transferrin receptor protein expression. RESULTS: The mRNA expression of 20 genes represented by 59 different cDNA clones significantly correlated to the log10IC50 values for the artemisinins, including genes encoding transferrin (TF), transferrin receptors 1 and 2 (TFRC, TFR2), cerulopasmin (CP), lactoferrin (LTF) and others. The ferroptosis inhibitor ferrostatin-1 and the iron chelator deferoxamine led to a significantly reduced cytotoxicity of artenimol, indicating ferroptosis as cell death mode. CONCLUSION: The numerous iron-related genes, whose expression correlated with the response to artemisinin derivatives speak in factor for the relevance of iron for the cytotoxic activity of these compounds. Treatment with ferroptosis-inducing agents such as artemisinin derivatives represents an attractive strategy for cancer therapy. Pre-therapeutic determination of iron-related genes may indicate tumor sensitivity to artemisinins. Ferroptosis induced by artemisinin-type drugs deserve further investigation for individualized tumor therapy.


