Platycoside FCAS# 314756-03-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 314756-03-1 | SDF | Download SDF |
| PubChem ID | 101048500.0 | Appearance | Powder |
| Formula | C47H76O20 | M.Wt | 961.09 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
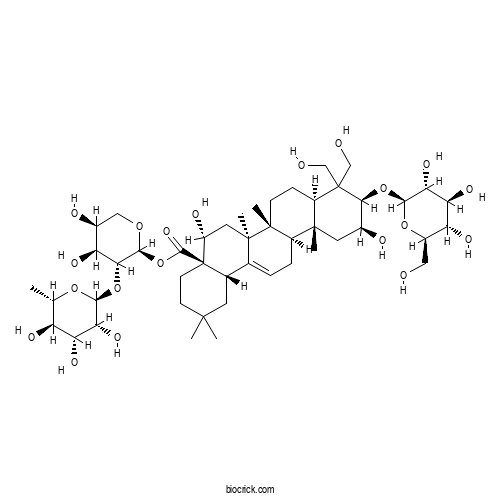
| Chemical Name | [(2S,3R,4S,5S)-4,5-dihydroxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl] (4aR,5R,6aR,6aS,6bR,8aR,10R,11S,12aR,14bS)-5,11-dihydroxy-9,9-bis(hydroxymethyl)-2,2,6a,6b,12a-pentamethyl-10-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylate | ||
| SMILES | CC1C(C(C(C(O1)OC2C(C(COC2OC(=O)C34CCC(CC3C5=CCC6C(C5(CC4O)C)(CCC7C6(CC(C(C7(CO)CO)OC8C(C(C(C(O8)CO)O)O)O)O)C)C)(C)C)O)O)O)O)O | ||
| Standard InChIKey | QIMBOUOMXGXUQK-HQQCROKPSA-N | ||
| Standard InChI | InChI=1S/C47H76O20/c1-20-29(54)32(57)34(59)38(63-20)65-36-30(55)24(52)17-62-40(36)67-41(61)47-12-11-42(2,3)13-22(47)21-7-8-26-43(4)14-23(51)37(66-39-35(60)33(58)31(56)25(16-48)64-39)46(18-49,19-50)27(43)9-10-44(26,5)45(21,6)15-28(47)53/h7,20,22-40,48-60H,8-19H2,1-6H3/t20-,22-,23-,24-,25+,26+,27+,28+,29-,30-,31+,32+,33-,34+,35+,36+,37-,38-,39-,40-,43+,44+,45+,47+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Platycoside F Dilution Calculator

Platycoside F Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0405 mL | 5.2024 mL | 10.4049 mL | 20.8097 mL | 26.0121 mL |
| 5 mM | 0.2081 mL | 1.0405 mL | 2.081 mL | 4.1619 mL | 5.2024 mL |
| 10 mM | 0.104 mL | 0.5202 mL | 1.0405 mL | 2.081 mL | 2.6012 mL |
| 50 mM | 0.0208 mL | 0.104 mL | 0.2081 mL | 0.4162 mL | 0.5202 mL |
| 100 mM | 0.0104 mL | 0.052 mL | 0.104 mL | 0.2081 mL | 0.2601 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6',6''- dimethyl glycyrrhizinate
Catalog No.:BCX0826
CAS No.:114006-81-4
- Heptasaccharide
Catalog No.:BCX0825
CAS No.:121591-98-8
- Licorice glycoside C2
Catalog No.:BCX0824
CAS No.:202657-55-4
- Picfeltarraegenin I
Catalog No.:BCX0823
CAS No.:82145-63-9
- 6''- methyl glycyrrhizinate
Catalog No.:BCX0822
CAS No.:1186016-30-7
- Tribuloside
Catalog No.:BCX0821
CAS No.:22153-44-2
- (25R)-26-O-β-D-Glucopyranosyl-22-hydroxy-5β-furost-3β,26-diol-3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside
Catalog No.:BCX0820
CAS No.:897386-27-5
- Coreoside B
Catalog No.:BCX0819
CAS No.:1580464-83-0
- Xylohexaose
Catalog No.:BCX0818
CAS No.:49694-21-5
- Hirudonucleodisulfide A
Catalog No.:BCX0817
CAS No.:1072789-37-7
- Xylopentaose
Catalog No.:BCX0816
CAS No.:49694-20-4
- Kuwanon U
Catalog No.:BCX0815
CAS No.:123702-95-4
- Quercetin 3-O-β-D-Glucuronide 6''-Methyl Ester
Catalog No.:BCX0828
CAS No.:79543-28-5
- 4′′,5′′-dehydroisopsoralidin
Catalog No.:BCX0829
CAS No.:65639-51-2
- Paeonoside
Catalog No.:BCX0830
CAS No.:20309-70-0
- Artemisitene
Catalog No.:BCX0831
CAS No.:101020-89-7
- 6''-O-Acetyldaidzin
Catalog No.:BCX0832
CAS No.:71385-83-6
- Aquilarone B
Catalog No.:BCX0833
CAS No.:1404479-45-3
- (S)-Goitrin
Catalog No.:BCX0834
CAS No.:500-12-9
- 6''-O-acetyl-saikosaponin B2
Catalog No.:BCX0835
CAS No.:104121-82-6
- 4'-Methoxyisoagarotetrol
Catalog No.:BCX0836
CAS No.:104901-10-2
- 8-epi-helenalin
Catalog No.:BCX0837
CAS No.:97643-91-9
- 4H-xanthalongin
Catalog No.:BCX0838
CAS No.:72843-22-2
- 2'-Hydroxylisoagarotetrol
Catalog No.:BCX0839
CAS No.:104926-77-4
Exploring the effects of different processing techniques on the composition and biological activity of Platycodon grandiflorus (Jacq.) A.DC. by metabonomics and pharmacologic design.[Pubmed:35038566]
J Ethnopharmacol. 2022 May 10;289:114991.
ETHNOPHARMACOLOGICAL RELEVANCE: Platycodon grandiflorus (Jacq.) A.DC. (PG) is a common natural medicine with a history of thousands of years. The processing products were mainly recorded as raw, honey-processed, wine-fried, yellow-fried, and bran-fried PG, which were respectively used for different clinical purposes. Therefore, it is necessary to study the chemical composition and pharmacological activity of PG after processing. AIM OF THE STUDY: To explore the effects of different processing methods on the composition and biological activity of PG using metabonomics and pharmacologic design. MATERIALS AND METHODS: UPLC-QTOF-MS combined with multivariate statistical analysis was used to identify different metabolites before and after the processing of PG. Network pharmacology was used to construct the metabolite-target-disease network. CCK-8 assay, flow cytometry, and western blotting were used to detect cell viability, apoptosis, and the expression of related proteins, respectively. RESULT: A total of 43 differentially expressed metabolites (VIP >10) were detected and identified in the analyzed groups. Based on their chemical nature, these metabolites were divided into five categories, namely, saccharolipids, flavonoid glycosides, alkynes, saponins, and lipids (including fatty acids, phospholipids, fatty aldehydes, and sterols). The content of lipids in the five processed groups (CH, FC, JZ, MZI, and MZG) was found to be higher than that in raw PG. In particular, the processing approaches explored herein increased the contents of many phospholipids, such as, glycerophosphoinositols, phosphatidic acids, and lysophosphatidyle.thanolamines. The 8 metabolites were found by venn diagram to distinguish different processed products (metabolites 2, 6, 19, 20, 21, 26, 28, and 38). The results of network pharmacology analysis showed that the primary anti-cancer targets of 43 metabolites of PG processing products are PIK3CA, Akt, and STAT3, and based on CCK-8 assay, MZI has a significant killing effect on A549 cells, compared to other processing techniques. Moreover, flow cytometry analysis showed that the cells treated with MZI exhibit significantly increased cell apoptosis, and that the effect is dose-dependent. Finally, the western blots performed herein demonstrated that the MZI effectively inhibits the expression of p-Akt and p-STAT3, which is consistent with the network pharmacology results. CONCLUSION: Depending on the processing technique, the contents of 43 different metabolites in PG were varied significantly. Specifically, the contents of phospholipids and fatty acids increase, whereas the contents of large Mw saponins decrease. Compared to the other investigated processing methods, MZI increases the potential of PG in inducing cell apoptosis and inhibiting cell proliferation by affecting the Akt and STAT3 signaling pathways. The increased levels of 3-O-beta-glucopyranosyl polygalacic acid and Platycoside F after honey-frying confirm these results.
Components study on antitussive effect and holistic mechanism of Platycodonis Radix based on spectrum-effect relationship and metabonomics analysis.[Pubmed:33872929]
J Chromatogr B Analyt Technol Biomed Life Sci. 2021 Apr 2;1173:122680.
The antitussive effect of Platycodonis Radix is closely related to the components in saponins fraction of Platycodonis Radix extract (SFPRE); however, these active components and their holistic mechanism remain unknown. Hence, a new method by integrating spectrum-effect relationship analysis with metabolomics analysis was applied to study the active components and their holistic mechanism simultaneously. For spectrum-effect relationship analysis, chemical fingerprints of ten batches of SFPRE were developed using UHPLC-LTQ-Orbitrap MS(n); antitussive effect were evaluated using a classic mice-cough model induced by ammonia liquor. Spectrum-effect relationship was analyzed by partial least squares regression (PLSR) analysis. For metabolomics analysis, the altered metabolites related to cough in serum were identified by UHPLC-Q-TOF/MS and orthogonal partial least squares-discriminant analysis (OPLS-DA); metabolic pathway analysis was depended on MetaboAnalyst 4.0, KEGG database, METLIN database and HMDB database. Our findings showed that 10 identified components of Polygalacin D (peak 26), Deapio-platycodin D (peak 21), Platycodin D (peak 23), beta-Gentiotriosyl platycodigenin (peak 37), Platycoside G3 (peak 17), Platycoside C (peak 25), Platycodin D3 (peak 16), 3-O-beta-D-glucopyranosyl platycodigenin (peak 33), Platycoside F (peak 19) and 3''-O-acetyl platycodin D3 (peak 15), and 2 unidentified components (peak 45 and 44) possessed antitussive effects. The metabolomics analysis result showed that 19 metabolites were potential biomarkers related to the cough, 16 of which could be restored to normal levels by SFPRE. These biomarkers were involved in arachidonic acid metabolism, linoleic acid metabolism and glycerophospholipid metabolism. The current study may facilitate the development of antitussive medicines with fewer side-effects based on Platycodonis Radix.
Platycoside O, a new triterpenoid saponin from the roots of Platycodon grandiflorum.[Pubmed:21617591]
Molecules. 2011 May 26;16(6):4371-8.
A new unusual minor triterpenoid saponin, platycoside O (1), was isolated from the 75% EtOH extract obtained from the roots of Platycodon grandiflorum, together with four known saponins: platycoside M-3 (2), platycoside J (3), Platycoside F (4) and platycoside B (5). The structure of 1 was determined as 3-O-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranosyl-2beta,3beta,16alpha,23-tetrahydroxyolean-12-en-24-methoxyl, 24-oxo-28-oic acid 28-O-beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl-(1-->2)-alpha-L-arabinopyranoside on the basis of spectral analysis and chemical evidence.
Five new triterpenoid saponins from the roots of Platycodon grandiflorum.[Pubmed:16595965]
Chem Pharm Bull (Tokyo). 2006 Apr;54(4):557-60.
Five new triterpenoid saponins, platycoside H [3-O-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranosyl-2beta,3beta,16alpha,23-tetrahydroxyolean-12-en-28-oic acid 28-O-beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl-(1-->2)-alpha-L-arabinopyranoside], platycoside I [3-O-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranosyl-2beta,3beta,16alpha,23-tetrahydroxyolean-12-en-28-oic acid 28-O-beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl-(1-->2)-alpha-L-arabinopyranoside], platycoside J [3-O-beta-D-glucopyranosyl-2beta,3beta,16alpha,23-tetrahydroxyolean-12-en-28-oic acid 28-O-beta-D-xylopyranosyl-(1-->4)-alpha-L-rhamnopyranosyl-(1-->2)-alpha-L-arabinopyranoside], platycoside K [3-O-beta-D-glucopyranosyl-(1-->3)-beta-D-glucopyranosyl-2beta,3beta,16alpha,23,24-pentahydroxyolean-12-en-28-oic acid], and platycoside L [3-O-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranosyl-2beta,3beta,16alpha,23,24-pentahydroxyolean-12-en-28 oic acid], and three known triterpenoid saponins, Platycoside F, platycoside B, and platycoside C, were isolated from the roots of Platycodon grandiflorum A. DC. Their chemical structures were elucidated on the basis of their spectral data and chemical evidence.


