PaeonosideCAS# 20309-70-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20309-70-0 | SDF | Download SDF |
| PubChem ID | 442924.0 | Appearance | Powder |
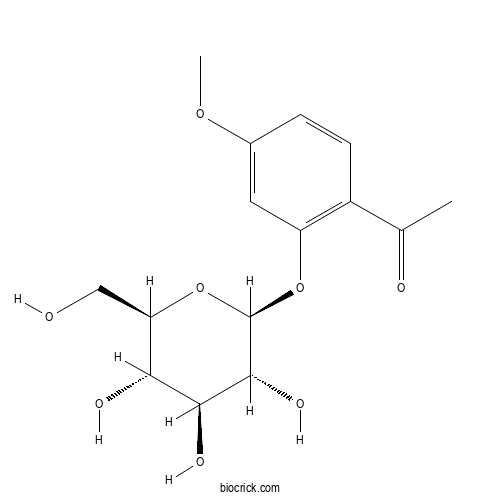
| Formula | C15H20O8 | M.Wt | 328.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-[4-methoxy-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]ethanone | ||
| SMILES | CC(=O)C1=C(C=C(C=C1)OC)OC2C(C(C(C(O2)CO)O)O)O | ||
| Standard InChIKey | AVIUTYMRHHBXPB-UXXRCYHCSA-N | ||
| Standard InChI | InChI=1S/C15H20O8/c1-7(17)9-4-3-8(21-2)5-10(9)22-15-14(20)13(19)12(18)11(6-16)23-15/h3-5,11-16,18-20H,6H2,1-2H3/t11-,12-,13+,14-,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Paeonoside Dilution Calculator

Paeonoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0459 mL | 15.2295 mL | 30.459 mL | 60.918 mL | 76.1475 mL |
| 5 mM | 0.6092 mL | 3.0459 mL | 6.0918 mL | 12.1836 mL | 15.2295 mL |
| 10 mM | 0.3046 mL | 1.523 mL | 3.0459 mL | 6.0918 mL | 7.6148 mL |
| 50 mM | 0.0609 mL | 0.3046 mL | 0.6092 mL | 1.2184 mL | 1.523 mL |
| 100 mM | 0.0305 mL | 0.1523 mL | 0.3046 mL | 0.6092 mL | 0.7615 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4′′,5′′-dehydroisopsoralidin
Catalog No.:BCX0829
CAS No.:65639-51-2
- Quercetin 3-O-β-D-Glucuronide 6''-Methyl Ester
Catalog No.:BCX0828
CAS No.:79543-28-5
- Platycoside F
Catalog No.:BCX0827
CAS No.:314756-03-1
- 6',6''- dimethyl glycyrrhizinate
Catalog No.:BCX0826
CAS No.:114006-81-4
- Heptasaccharide
Catalog No.:BCX0825
CAS No.:121591-98-8
- Licorice glycoside C2
Catalog No.:BCX0824
CAS No.:202657-55-4
- Picfeltarraegenin I
Catalog No.:BCX0823
CAS No.:82145-63-9
- 6''- methyl glycyrrhizinate
Catalog No.:BCX0822
CAS No.:1186016-30-7
- Tribuloside
Catalog No.:BCX0821
CAS No.:22153-44-2
- (25R)-26-O-β-D-Glucopyranosyl-22-hydroxy-5β-furost-3β,26-diol-3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside
Catalog No.:BCX0820
CAS No.:897386-27-5
- Coreoside B
Catalog No.:BCX0819
CAS No.:1580464-83-0
- Xylohexaose
Catalog No.:BCX0818
CAS No.:49694-21-5
- Artemisitene
Catalog No.:BCX0831
CAS No.:101020-89-7
- 6''-O-Acetyldaidzin
Catalog No.:BCX0832
CAS No.:71385-83-6
- Aquilarone B
Catalog No.:BCX0833
CAS No.:1404479-45-3
- (S)-Goitrin
Catalog No.:BCX0834
CAS No.:500-12-9
- 6''-O-acetyl-saikosaponin B2
Catalog No.:BCX0835
CAS No.:104121-82-6
- 4'-Methoxyisoagarotetrol
Catalog No.:BCX0836
CAS No.:104901-10-2
- 8-epi-helenalin
Catalog No.:BCX0837
CAS No.:97643-91-9
- 4H-xanthalongin
Catalog No.:BCX0838
CAS No.:72843-22-2
- 2'-Hydroxylisoagarotetrol
Catalog No.:BCX0839
CAS No.:104926-77-4
- Aquilarone C
Catalog No.:BCX0840
CAS No.:1404479-46-4
- (6S,7S,8S)-5,6,7,8-Tetrahydro-6,7,8-trihydroxy-2-(2-phenylethyl)-4H-1-benzopyran-4-one
Catalog No.:BCX0841
CAS No.:2803559-82-0
- 6-Epiagarotetrol
Catalog No.:BCX0842
CAS No.:2580359-99-3
Biological Mechanisms of Paeonoside in the Differentiation of Pre-Osteoblasts and the Formation of Mineralized Nodules.[Pubmed:34199016]
Int J Mol Sci. 2021 Jun 27;22(13):6899.
Paeonia suffruticosa is a magnificent and long-lived woody plant that has traditionally been used to treat various diseases including inflammatory, neurological, cancer, and cardiovascular diseases. In the present study, we demonstrated the biological mechanisms of Paeonoside (PASI) isolated from the dried roots of P. suffruticosa in pre-osteoblasts. Herein, we found that PASI has no cytotoxic effects on pre-osteoblasts. Migration assay showed that PASI promoted wound healing and transmigration in osteoblast differentiation. PASI increased early osteoblast differentiation and mineralized nodule formation. In addition, PASI enhanced the expression of Wnt3a and bone morphogenetic protein 2 (BMP2) and activated their downstream molecules, Smad1/5/8 and beta-catenin, leading to increases in runt-related transcription factor 2 (RUNX2) expression during osteoblast differentiation. Furthermore, PASI-mediated osteoblast differentiation was attenuated by inhibiting the BMP2 and Wnt3a pathways, which was accompanied by reduction in the expression of RUNX2 in the nucleus. Taken together, our findings provide evidence that PASI enhances osteoblast differentiation and mineralized nodules by regulating RUNX2 expression through the BMP2 and Wnt3a pathways, suggesting a potential role for PASI targeting osteoblasts to treat bone diseases including osteoporosis and periodontitis.
Polyphenols and neuroprotection against ischemia and neurodegeneration.[Pubmed:22070681]
Mini Rev Med Chem. 2011 Dec;11(14):1222-38.
Neuroprotection of polyphenols in medical plants is getting attention in the world. Scutellaria baicalensis, paeonia veitchii and paeonia suffruticosa have been extensively studied in the last 10 years and show multi-function. They are neuroprotectants, antioxidants, anti-inflammatory and antithrombic agents as well as vasoconstriction inhibitors and amyloid-peptide (Abeta) cleaners by means of their polyphenols: baicalin, baicalein, wogonin (in scutellaria), and paeonol, Paeonoside, paeoniflorin (PF) and 1, 2, 3, 4, 6-Penta-O-galloyl-beta-D-glucose (PGG) (in paeonia veitchii and paeonia suffruticosa). Other 4 medical plants: astragali, ligusticum wallichii, angelica sinensis and carthamus tinctorius (saffron) have been the major medicines to treat ischemia for hundreds of years in China, Korea and Japan. Our recent experimental studies demonstrated the neuroprotective efficacy of the combination of these phyotmedicines on mitigating brain infarction and global ischemia as well as preventing the neurodegeneration following ischemia. Owing to their multi-function, including improving cerebral blood circulation, they therefore have the potential to alleviate the symptoms of degenerative diseases, Alzheimer's disease (AD) and Parkinson's disease (PD). Pharmacology of the 7 herbs and their major relative polyphenols is depicted in the article.
Determination of glycosides and sugars in Moutan Cortex by capillary electrophoresis with electrochemical detection.[Pubmed:16343840]
J Pharm Biomed Anal. 2006 Apr 11;41(1):129-34.
A method based on capillary electrophoresis with electrochemical detection has been developed for the separation and determination of paeoniflorin, sucrose, Paeonoside, glucose, and fructose in Moutan Cortex for the first time. Effects of several important factors such as the concentration of NaOH, separation voltage, injection time, and detection potential were investigated to acquire the optimum conditions. The detection electrode was a 300 microm diameter copper disc electrode at a working potential of +0.60 V (versus saturated calomel electrode (SCE)). The five analytes can be well-separated within 12 min in a 40 cm length fused silica capillary at a separation voltage of 12 kV in a 75 mM NaOH aqueous solution. The relation between peak current and analyte concentration was linear over about 3 orders of magnitude with detection limits (S/N = 3) ranging from 0.9 to 1.3 microM for all analytes. The proposed method has been successfully applied to monitor glycoside and sugar contents in the real plant samples with satisfactory assay results.
Protective constituents against sepsis in mice from the root cortex of Paeonia suffruticosa.[Pubmed:15595414]
Arch Pharm Res. 2004 Nov;27(11):1123-6.
The bioassay-guided fractionation of protective agents against sepsis-induced lethality from the root cortex of Paeonia suffruticosa ANDREWS (Ranunculaceae) led to the isolation of eight known compounds: paeonol (1), 2,5-dihydroxy-4-methoxyacetophenone (2), acetovanillone (3), Paeonoside (4), paeoniflorin (5), oxypaeoniflorin (6), apioPaeonoside (7), and methyl 3-hydroxy-4-methoxybenzoate (8). Among them, 3 showed the highest survival rate (100% with a dose of 30 mg/kg versus 17% for the control experiment) and reduced alanine aminotransferase level to be a half of the control value on the sepsis model induced by lipopolysaccharide/D-galactosamine.
[Flavoneglycosides in the flowers of Paeonia arborea and P. suffruticosa].[Pubmed:24504864]
Planta. 1969 Jun;88(2):154-6.
Three glycosides have been isolated fromPaeonia arborea: kaempferol-3-beta-glucoside-7-beta-glucoside (Paeonoside), apigenin-7-beta-glucoside, and apigenin-7-rhamnoglucoside (Rhoifolin).Paeonia suffruticosa also contains these three compounds but its main glycoside is kaempferol-3-beta-glucoside (astragalin), which is present inPaeonia arborea only in traces.


