KoenigineCAS# 28513-33-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28513-33-9 | SDF | Download SDF |
| PubChem ID | 5318825 | Appearance | Powder |
| Formula | C19H19NO3 | M.Wt | 309.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
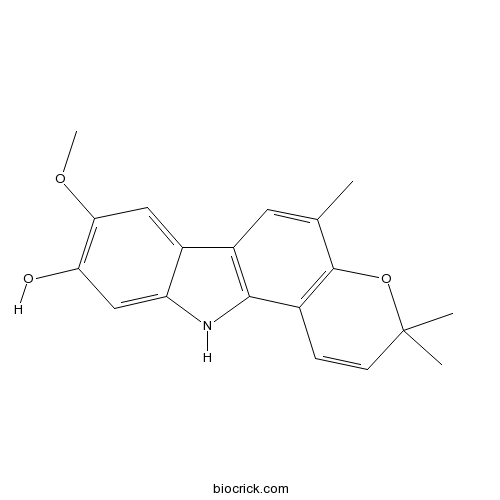
| Chemical Name | 8-methoxy-3,3,5-trimethyl-11H-pyrano[3,2-a]carbazol-9-ol | ||
| SMILES | CC1=CC2=C(C3=C1OC(C=C3)(C)C)NC4=CC(=C(C=C42)OC)O | ||
| Standard InChIKey | CZZZOTXCAIDYOZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H19NO3/c1-10-7-13-12-8-16(22-4)15(21)9-14(12)20-17(13)11-5-6-19(2,3)23-18(10)11/h5-9,20-21H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Koenigine Dilution Calculator

Koenigine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2321 mL | 16.1603 mL | 32.3206 mL | 64.6412 mL | 80.8016 mL |
| 5 mM | 0.6464 mL | 3.2321 mL | 6.4641 mL | 12.9282 mL | 16.1603 mL |
| 10 mM | 0.3232 mL | 1.616 mL | 3.2321 mL | 6.4641 mL | 8.0802 mL |
| 50 mM | 0.0646 mL | 0.3232 mL | 0.6464 mL | 1.2928 mL | 1.616 mL |
| 100 mM | 0.0323 mL | 0.1616 mL | 0.3232 mL | 0.6464 mL | 0.808 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Trigothysoid O
Catalog No.:BCN9531
CAS No.:1501943-09-4
- Bletillatin A
Catalog No.:BCN9530
CAS No.:2387570-11-6
- 3,5,8,3'-Tetramethoxy-6,7,4',5'-bis(methylenedioxy)flavone
Catalog No.:BCN9529
CAS No.:82668-93-7
- Severibuxine
Catalog No.:BCN9528
CAS No.:219998-24-0
- 4'-O-Methylatalantoflavone
Catalog No.:BCN9527
CAS No.:1205687-49-5
- Buxifoliadine B
Catalog No.:BCN9526
CAS No.:263007-66-5
- 22-Hydroxycyclolaudenol
Catalog No.:BCN9525
CAS No.:2077090-18-5
- 6-Demethoxyleptostachyol acetate
Catalog No.:BCN9524
CAS No.:126298-48-4
- 5'-Demethoxycyrtonesin A
Catalog No.:BCN9523
CAS No.:1179524-22-1
- Dihydrokaempferol 3-O-glucoside
Catalog No.:BCN9522
CAS No.:31049-08-8
- Koenigicine
Catalog No.:BCN9521
CAS No.:24123-92-0
- Platachromone B
Catalog No.:BCN9520
CAS No.:1606149-63-6
- Murrastinine C
Catalog No.:BCN9533
CAS No.:20105-20-8
- Murrayamine C
Catalog No.:BCN9534
CAS No.:73385-58-7
- Quercetin 3,7-di-O-rhamnoside
Catalog No.:BCN9535
CAS No.:28638-13-3
- 1-Prenyl-2-methoxy-6-formyl-8-hydroxy-9H-carbazole
Catalog No.:BCN9536
CAS No.:484678-79-7
- 5-Hydroxy-3,6,7,8,3'-pentamethoxy-4',5'-methylenedioxyflavone
Catalog No.:BCN9537
CAS No.:82669-01-0
- Buxifoliadine C
Catalog No.:BCN9538
CAS No.:263007-67-6
- 8,8''-Biskoenigine
Catalog No.:BCN9539
CAS No.:477890-82-7
- Atalaphylline
Catalog No.:BCN9540
CAS No.:28233-35-4
- Atalafoline
Catalog No.:BCN9541
CAS No.:107259-49-4
- (1R,6R,9R)-6,9,11-Trihydroxy-4,7-megastigmadien-3-one 11-O-glucoside
Catalog No.:BCN9542
CAS No.:289914-68-7
- 4"-O-Acetylastilbin
Catalog No.:BCN9543
CAS No.:1298135-49-5
- 5,5'-Dihydroxy-3,8,3',4'-tetramethoxy-6,7-methylenedioxyflavone
Catalog No.:BCN9544
CAS No.:82668-96-0
Synthesis of 1,1'- and 2,2'-Bicarbazole Alkaloids by Iron(III)-Catalyzed Oxidative Coupling of 2- and 1-Hydroxycarbazoles.[Pubmed:29024097]
Chemistry. 2018 Jan 9;24(2):458-470.
We describe the synthesis of 1,1'- and 2,2'-bicarbazoles by oxidative homocoupling of 2- and 1-hydroxycarbazoles. The oxidative coupling using catalytic amounts of F16 PcFe can be applied to both groups of substrates. Although F16 PcFe generally provides the best yields for the synthesis of 1,1'-bicarbazoles, di-tert-butyl peroxide affords better results for the 2,2'-bicarbazoles. In our study, we have achieved the first syntheses of the biscarbalexines A-C, bisglybomine B, 2,2'-dihydroxy-7,7'-dimethoxy-3,3'-dimethyl-1,1'-bicarbazole, bispyrayafoline C, and bisisomahanine. The iron-catalyzed coupling of Koenigine led to an improved synthesis of 8,8''-bisKoenigine and afforded an unprecedented decacylic product. Oxidative coupling of 1-hydroxycarbazoles led to bisclausenol, and to the first total syntheses of bismurrayafoline B and D.
Pyranocarbazoles from Murraya koenigii (L.) Spreng. as antimicrobial agents.[Pubmed:28368664]
Nat Prod Res. 2018 Feb;32(4):430-434.
The bioassay guided fractionation of methanolic extract of Murraya koenigii (L.) Spreng. leaves resulted in the isolation of seven pyranocarbazoles. These were evaluated against four bacterial strains and ten Candida sp. including two matched pair of fluconazole sensitive/resistant clinical isolates. Out of seven, three i.e. Koenine (mk279), Koenigine (mk309) and Mahanine (mk347) exhibited significant antibacterial activity MIC90 3.12-12.5 mug/mL against bacterial strains Streptococcus aureus and Klebsiella pneumonia compared with standard drug Kanamycin MIC90 12.5 mug/mL. However, only mk309 was found active against variety of Candida species MIC90 12.5-100 mug/mL. It was observed that hydroxylation at C-6 and C-7 positions in the studied pyranocarbazoles activate the bioactivity. Simultaneously, decrease in Log P value compares with -H and -O-CH3 substituted derivatives. The study is focused on selective antifungal and antibacterial activity of pyranocarbazoles on bacterial strains S. aureus, K. pneumonia and variety of Candida species with structure activity relationship observations.
Two new carbazole alkaloids from Murraya koenigii.[Pubmed:12662104]
J Nat Prod. 2003 Mar;66(3):416-8.
Two new carbazole alkaloids named murrayanine (1) and 8,8' '-bisKoenigine (2) were isolated from Murraya koenigii. The structure elucidations for 1 and 2 were carried out on the basis of 1D and 2D NMR experiments. Compound 1 was a novel carbazole alkaloid with a rare phenylpropanyl substitution. Compound 2 was a symmetrical dimer of the carbazole alkaloid Koenigine and showed antiosteoporotic activity in the CAT-B model with IC(50) 1.3 microg/mL. The synthesis of 2 from Koenigine was carried out through oxidative coupling using a solid state reaction.
[Study on carbazole alkaloids of Murraya microphylla].[Pubmed:12571921]
Zhong Yao Cai. 1999 Sep;22(9):458-60.
Four carbazole alkaloids were isolated from the methanol extract of Murraya microphylla. Their structures were identified by spectral analysis and chemical evidence, which were identified as Koenigine, bis-6-hydroxy-7-methoxygirinimbine, girinimbine and mukonicine. All the compounds were discovered from this plant for the first time, and bis-6-hydroxy-7-methoxygirinimbine was a new compound.


