Lantanilic acidCAS# 60657-41-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60657-41-2 | SDF | Download SDF |
| PubChem ID | 101316804 | Appearance | Powder |
| Formula | C35H52O6 | M.Wt | 568.8 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
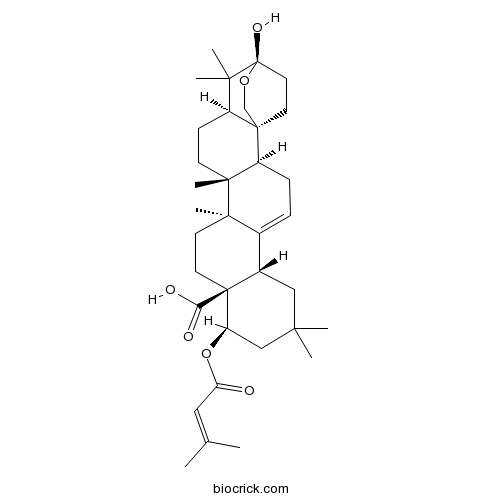
| Chemical Name | (1S,2S,6S,10R,11S,14S,15R,18R,20S)-20-hydroxy-8,8,14,15,19,19-hexamethyl-10-(3-methylbut-2-enoyloxy)-21-oxahexacyclo[18.2.2.01,18.02,15.05,14.06,11]tetracos-4-ene-11-carboxylic acid | ||
| SMILES | CC(=CC(=O)OC1CC(CC2C1(CCC3(C2=CCC4C3(CCC5C46CCC(C5(C)C)(OC6)O)C)C)C(=O)O)(C)C)C | ||
| Standard InChIKey | HGMVESCHSMFWDD-ZUQXEZLCSA-N | ||
| Standard InChI | InChI=1S/C35H52O6/c1-21(2)17-27(36)41-26-19-29(3,4)18-23-22-9-10-25-32(8,31(22,7)13-15-34(23,26)28(37)38)12-11-24-30(5,6)35(39)16-14-33(24,25)20-40-35/h9,17,23-26,39H,10-16,18-20H2,1-8H3,(H,37,38)/t23-,24-,25-,26+,31+,32+,33+,34-,35-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Lantanilic acid Dilution Calculator

Lantanilic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7581 mL | 8.7904 mL | 17.5809 mL | 35.1617 mL | 43.9522 mL |
| 5 mM | 0.3516 mL | 1.7581 mL | 3.5162 mL | 7.0323 mL | 8.7904 mL |
| 10 mM | 0.1758 mL | 0.879 mL | 1.7581 mL | 3.5162 mL | 4.3952 mL |
| 50 mM | 0.0352 mL | 0.1758 mL | 0.3516 mL | 0.7032 mL | 0.879 mL |
| 100 mM | 0.0176 mL | 0.0879 mL | 0.1758 mL | 0.3516 mL | 0.4395 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cucurbitacin A 2-O-β-D-glucopyranoside
Catalog No.:BCN9327
CAS No.:1135141-76-2
- 4a-Demethoxysampsone B
Catalog No.:BCN9326
CAS No.:1292798-98-1
- Icterogenin
Catalog No.:BCN9325
CAS No.:561-47-7
- Phoyunnanin E
Catalog No.:BCN9324
CAS No.:886747-60-0
- Leeaoside
Catalog No.:BCN9323
CAS No.:1015063-56-5
- Hymexelsin
Catalog No.:BCN9322
CAS No.:117842-09-8
- Giffonin P
Catalog No.:BCN9321
CAS No.:1830306-93-8
- Dicliripariside A
Catalog No.:BCN9320
CAS No.:491613-65-1
- Jinflexin D
Catalog No.:BCN9319
CAS No.:2055155-78-5
- 10-Hydroxyligstroside
Catalog No.:BCN9318
CAS No.:35897-94-0
- 3-epi-Isocucurbitacin B
Catalog No.:BCN9317
CAS No.:89647-62-1
- Flazin
Catalog No.:BCN9316
CAS No.:100041-05-2
- Methyl lucidenate E2
Catalog No.:BCN9329
CAS No.:98665-12-4
- Hancokinol
Catalog No.:BCN9330
CAS No.:132294-77-0
- 7β-Hydroxycucurbitacin B
Catalog No.:BCN9331
CAS No.:1135141-79-5
- 3α,30-Diacetoxy-12α-hydroxy-23-oxoeupha-7,24-dien-21,16β-olid-28-oic acid 28-O-β-D-glucopyranosyl ester
Catalog No.:BCN9332
CAS No.:215160-96-6
- Sampsone B
Catalog No.:BCN9333
CAS No.:1309125-17-4
- Aureusidin
Catalog No.:BCN9334
CAS No.:38216-54-5
- Thymol isobutyrate
Catalog No.:BCN9335
CAS No.:5451-67-2
- 1-Methyleffusol
Catalog No.:BCN9336
CAS No.:144106-78-5
- Jasnervoside C
Catalog No.:BCN9337
CAS No.:1622337-36-3
- Phochinenin G
Catalog No.:BCN9338
CAS No.:1070883-75-8
- Hancolupenone
Catalog No.:BCN9339
CAS No.:132746-04-4
- 8,9-Dehydro-7,9-diisobutyryloxythymol
Catalog No.:BCN9340
CAS No.:22518-03-2
Leishmanicidal Triterpenes from Lantana camara.[Pubmed:24827681]
Chem Biodivers. 2014 May;11(5):709-18.
Two new natural triterpenes, lantaninilic acid and lantoic acid, along with the known triterpenes lantadene A, and oleanolic, ursolic, betulinic, lantanolic, and camaric acid, were obtained from the aerial parts of Lantana camara through bioassay-guided isolation, monitoring the in vitro antileishmanial activity against promastigotes of Leishmania major. Oleanolic acid (3), ursolic acid (4), lantadene A (5), and Lantanilic acid (7) showed significant leishmanicidal activities with IC50 values of 53.0, 12.4, 20.4, and 21.3 muM, respectively. The IC50 value of ursolic acid (4; 12.4 muM) was found to be comparable with that of the standard drugs, pentamidine (IC50 15.0 muM) and amphotericin B (IC50 0.31 muM). The in vitro activities of L. camara and its constituents against promastigotes of Leishmania major are reported here for the first time.
A new pentacyclic triterpenoid from the leaves of Lantana montevidensis (Spreng.) Briq.[Pubmed:23961713]
Nat Prod Res. 2013;27(21):2046-52.
A new pentacyclic triterpenoid, 3beta,25-epoxy-3alpha,22beta,23alpha-trihydroxy-olean-12-en-28-oic acid (1), together with seven known compounds, including five triterpenoids, beta-amyrin (2), lantadene B (3), Lantanilic acid (4), lantanolic acid (5) and ursolic acid (6) in addition to beta-sitosterol (7) and benzoic acid (8) has been isolated from the leaves of Lantana montevidensis. Their chemical structures were elucidated by spectroscopic analysis and by comparison with the literature data and/or authentic samples. Compound 1 showed moderate to weak antibacterial activity against Staphylococcus aureus and Escherichia coli.
Pentacyclic triterpenoids from the aerial parts of Lantana camara and their nematicidal activity.[Pubmed:18816515]
Chem Biodivers. 2008 Sep;5(9):1856-66.
Two new olean-12-ene triterpenoids, camarolic acid (1) and lantrigloylic acid (2), have been isolated from the aerial parts of Lantana camara, along with ten known triterpenes, namely, camaric acid, lantanolic acid, Lantanilic acid, pomolic acid, camarinic acid, lantoic acid, camarin, lantacin, camarinin, and ursolic acid. The new compounds have been characterized as 3,25-epoxy-3alpha-hydroxy-22beta-{[(S)-3-hydroxy-2-methylidenebutanoyl]oxy}olean- 12-en-28-oic acid (1) and 3,25-epoxy-3alpha-hydroxy-22beta-[(3-methylbut-2-enoyl)oxy]olea-9(11),12-dien-28- oic acid (2) through spectroscopic studies and a chemical transformation. Seven of the constituents, namely pomolic acid, lantanolic acid, lantoic acid, camarin, lantacin, camarinin, and ursolic acid, were tested for nematicidal activity against root-knot nematode Meloidogyne incognita. Pomolic acid, lantanolic acid, and lantoic acid showed 100% mortality at 1 mg/ml concentration after 24 h, while camarin, lantacin, camarinin, and ursolic acid exhibited 100% mortality at this concentration after 48 h. These results are comparable to those obtained with the conventional nematicide furadan (100% mortality at 1 mg/ml concentration after 24 h).
Nematicidal natural products from the aerial parts of Lantana camara Linn.[Pubmed:16010828]
Nat Prod Res. 2005 Sep;19(6):609-13.
Lantanilic acid, camaric acid and oleanolic acid possessing nematicidal activity were isolated from the methanolic extract of the aerial parts of Lantana camara Linn. through bio-assay guided fractionation. These compounds exhibited 98%, 95% and 70% mortality respectively against root-knot nematode Meloidogyne incognita at 0.5% concentration. Conventional nematicide furadan showed 100% mortality at this concentration.
Oleanene constituents of Lantana cujabensis.[Pubmed:15158990]
Fitoterapia. 2004 Jun;75(3-4):327-31.
A new compound, 3beta,25-epoxy-3alpha-hydroxy-22beta-isobutanoyloxyolean-12-ene-28-oic acid (1), and two known triterpenoids Lantanilic acid (2) and camaric acid (3) were isolated from the stem and leaves of Lantana cujabensis. Their structures were elucidated by spectroscopic methods. The ethanol extracts did not show significant in vitro antiplasmodial activity against chloroquine-sensitive or resistant strains of Plasmodium falciparum.
Antitubercular activity of triterpenoids from Lippia turbinata.[Pubmed:11170663]
J Nat Prod. 2001 Jan;64(1):37-41.
Assay-guided fractionation of the antitubercular MeOH-CH(2)Cl(2) extract obtained from Lippia turbinata led to the isolation of four novel triterpenoids-3beta,25-epoxy-3alpha,21alpha-dihydroxy-22beta-(3-methylbut-2-en-1- oyloxy)olean-12-ene-28-oic acid (1); 3beta,25-epoxy-3alpha,21alpha-dihydroxy-22beta-angeloyloxyolean-12-ene-28-oic acid (2); 3beta,25-epoxy-3alpha,21alpha-dihydroxy-22beta-tigloyloxyolean-12-ene-28-oic acid (3); and 3beta,25-epoxy-3alpha-hydroxy-22beta-(2-methylbutan-1-oyloxy)olean-12-ene-28-oic acid (4)-together with the known triterpenoids Lantanilic acid (5), camaric acid (6), lantanolic acid (7), and rehmannic acid (8). The MIC values of 1-8 for growth inhibition of Mycobacterium tuberculosis were determined in the radiorespirometric BACTEC system.
[Studies on the chemical constituents of the leaves of Lantana camara].[Pubmed:8328268]
Yao Xue Xue Bao. 1993;28(1):35-9.
Six compounds were isolated from leaves of Lantana camara. On the basis of chemical and spectral (UV, IR, EI-MS, 1HNMR 13CNMR) analysis, they were identified as oleanonic acid (I), lantadene A (II), lantadene B (III), Lantanilic acid (IV), icterogenin (V) and 4',5-dihydroxy-3,7-dimethoxyflavone-4'-O-beta-D-glucopyranoside (VI). VI is a new compound named camaroside.


