Methyl piperateCAS# 6190-46-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6190-46-1 | SDF | Download SDF |
| PubChem ID | 9921021 | Appearance | Powder |
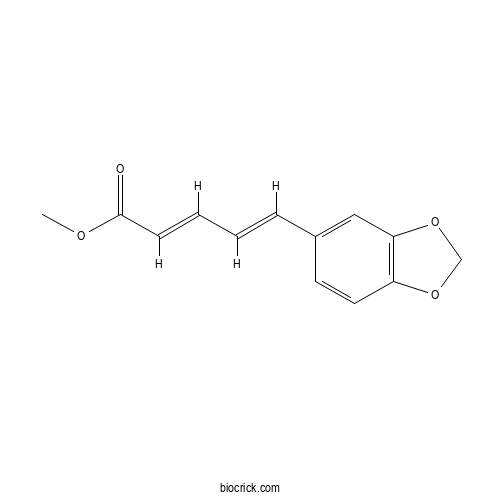
| Formula | C13H12O4 | M.Wt | 232.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (2E,4E)-5-(1,3-benzodioxol-5-yl)penta-2,4-dienoate | ||
| SMILES | COC(=O)C=CC=CC1=CC2=C(C=C1)OCO2 | ||
| Standard InChIKey | VOZJBFJHMHRLDN-ZUVMSYQZSA-N | ||
| Standard InChI | InChI=1S/C13H12O4/c1-15-13(14)5-3-2-4-10-6-7-11-12(8-10)17-9-16-11/h2-8H,9H2,1H3/b4-2+,5-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Methyl piperate Dilution Calculator

Methyl piperate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3066 mL | 21.5332 mL | 43.0663 mL | 86.1326 mL | 107.6658 mL |
| 5 mM | 0.8613 mL | 4.3066 mL | 8.6133 mL | 17.2265 mL | 21.5332 mL |
| 10 mM | 0.4307 mL | 2.1533 mL | 4.3066 mL | 8.6133 mL | 10.7666 mL |
| 50 mM | 0.0861 mL | 0.4307 mL | 0.8613 mL | 1.7227 mL | 2.1533 mL |
| 100 mM | 0.0431 mL | 0.2153 mL | 0.4307 mL | 0.8613 mL | 1.0767 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Periclymenosidic acid
Catalog No.:BCX0143
CAS No.:96681-56-0
- 4,7,9,9'-Tetrahydroxy-3,3'-dimethoxy-8,4'-oxyneolignan 7-O-beta-D-glucopyranoside
Catalog No.:BCX0142
CAS No.:182056-97-9
- Pipernonaline
Catalog No.:BCX0141
CAS No.:88660-10-0
- Pyrolaside B
Catalog No.:BCX0140
CAS No.:868632-32-0
- Jatamanvaltrate N
Catalog No.:BCX0139
CAS No.:1395056-08-2
- Crocin 3
Catalog No.:BCX0138
CAS No.:55750-85-1
- Alisol P
Catalog No.:BCX0137
CAS No.:1005191-19-4
- 3,3'-Dihydroxy-4',5,7-trimethoxyflavan
Catalog No.:BCX0136
CAS No.:97914-22-2
- Pyrocallianthaside A
Catalog No.:BCX0135
CAS No.:1004783-55-4
- (2R,4S)-3,4-Dihydro-4-hydroxy-2,7-dimethyl-1(2H)-naphthalenone
Catalog No.:BCX0134
CAS No.:1932291-56-9
- Pyrolaside A
Catalog No.:BCX0133
CAS No.:868632-29-5
- Jatairidoid B
Catalog No.:BCX0132
CAS No.:1393577-30-4
- 7-(Hydroxymethyl)-2-methyl-1,4-naphthalenedione
Catalog No.:BCX0145
CAS No.:145626-36-4
- 3-Hydroxy-4',5,7-trimethoxyflavan
Catalog No.:BCX0146
CAS No.:3143-21-3
- Jatairidoid A
Catalog No.:BCX0147
CAS No.:1393577-29-1
- 2,4,12-Octadecatrienoic acid isobutylamide
Catalog No.:BCX0148
CAS No.:151391-69-4
- Pyrolaside B analogue
Catalog No.:BCX0149
CAS No.:1053610-99-3
- Dehydropipernonaline
Catalog No.:BCX0150
CAS No.:107584-38-3
- threo-7,9,9'-Trihydroxy-3,3'-dimethoxy-8-O-4'-neolignan 4-O-beta-D-glucopyranoside
Catalog No.:BCX0151
CAS No.:1009838-88-3
- Justicidin B
Catalog No.:BCX0152
CAS No.:17951-19-8
- Chinensinaphthol methyl ether
Catalog No.:BCX0153
CAS No.:53965-11-0
- Justicidin D
Catalog No.:BCX0154
CAS No.:27041-98-1
- Isorinic acid
Catalog No.:BCX0155
CAS No.:145757-60-4
- Melliferone
Catalog No.:BCX0156
CAS No.:377724-68-0
Isolation and Characterization of Acetylcholinesterase Inhibitors from Piper longum and Binding Mode Predictions.[Pubmed:32668479]
Planta Med. 2020 Oct;86(15):1118-1124.
Restoration of cholinergic function is considered a rational approach to enhance cognitive performance. Acetylcholinesterase inhibitors are still the best therapeutic option for Alzheimer's disease. The fruits of Piper longum have been used in traditional medicines for the treatment of memory loss. It was demonstrated that the dichloromethane extract of these fruits is able to inhibit acetylcholinesterase. Thus, the aim of this study was to identify the contained acetylcholinesterase inhibitors. The active zones were presented via TLC-bioautography, and five compounds were isolated in the process of a bioassay-guided phytochemical investigation. Their structures were characterized as piperine, Methyl piperate, guineenisine, pipercide, and pellitorine using spectroscopy and spectrometry methods (UV, IR, MS, (1)H-, and (13)C-NMR). In vitro acetylcholinesterase inhibitory activities of the isolates and their IC(50) values were determined via a colorimetric assay. Three of them exhibited enzyme inhibitory activities, with piperine being the most potent compound (IC(50) of 0.3 mM). In order to investigate the binding mode of the tested compounds, docking studies were performed using the X-ray crystal structure of acetylcholinesterase from Tetronarce californica with the Protein Data Bank code 1EVE. The content of the active compounds in the extract was determined by a developed HPLC method. Piperine was present in the maximum quantity in the fruits (0.57%), whereas Methyl piperate contained the minimum content (0.10%).
Cytotoxic activity against small cell lung cancer cell line and chromatographic fingerprinting of six isolated compounds from the ethanolic extract of Benjakul.[Pubmed:25518296]
J Med Assoc Thai. 2014 Aug;97 Suppl 8:S70-5.
BACKGROUND: Benjakul, a Thai traditional herbal preparation, comnprises five plants: Piper chaba, Piper sarmentosum, Piper interruptum, Plumbago indica, and Zingiber officinale. It has widely been used to treat cancer patients in folk medicine in Thailand. Benjakul extract, and its isolated compounds should be investigated for cytotoxic activity and analysis isolated compounds from chemical fingerprinting. OBJECTIVE: To study cytotoxicity ofBenjakul extract and its isolatedpure compounds against human small cell lung cancer cell line (NCI-HI 688) and in normal human lungfibroblast cell line (MRC-5) and analysis the content ofisolated compounds for quality control of Benjakul extract. MATERIAL AND METHOD: Bioassay-guided fractionation was used for isolated active compounds from ethanolic extract of Benjakul. Cytotoxic activity was carried using the SRB assay. HPLC method was applied to analyze six isolated compound contentfrom Benjakul extract. RESULTS: The ethanolic extract ofBenjakul showed cytotoxicity against NCI-H1688 with IC50 value = 36.15+/-4.35 mug/ml. Hexane fraction as semi-separation by VLC showed the best cytotoxic activity (21.1 7+/-7.42 mug/ml). Six isolated compounds were identified as myristicin, plumbagin, Methyl piperate, 6-shogaol, 6-gingerol and piperine. Plumbagin exhibited the highest cytotoxic activity and 6-shogaol was the second most effective cytotoxic constituent (IC50 values = 1.41+/-0.01 and 6.45+/-0.19 mug/ml, respectively). Piperine showed the highest content in both ofHPLC analysis and column chromatography separation. CONCLUSION: Benjakul extract exhibited cytotoxicity against NCI-HI 688. Plumbagin and 6-shogaol are bioactive markers for cytotoxicity against this small cell lung cancer cell line. Chromatographic fingerprinting can be used to analyze six cytotoxic compounds isolatedfrom the ethanolic extract ofBenjakul.
Development of piperic acid derivatives from Piper nigrum as UV protection agents.[Pubmed:25471519]
Pharm Biol. 2015 Apr;53(4):477-82.
CONTEXT: There is a need for the discovery of novel natural and semi-synthetic sunscreen that is safe and effective. Piperine has a UV absorption band of 230-400 nm with high molar absorptivity. This compound has a high potential to be developed to sunscreen. OBJECTIVE: This study develops new UV protection compounds from piperine by using chemical synthesis. MATERIALS AND METHODS: Piperine was isolated from Piper nigrum L. (Piperaceae) fruits, converted to piperic acid by alkaline hydrolysis, and prepared as ester derivatives by chemical synthesis. The piperate derivatives were prepared as 5% o/w emulsion, and the SPF values were evaluated. The best compound was submitted to cytotoxicity test using MTT assay. RESULTS: Piperic acid was prepared in 86.96% yield. Next, piperic acid was reacted with alcohols using Steglich reaction to obtain Methyl piperate, ethyl piperate, propyl piperate, isopropyl piperate, and isobutyl piperate in 62.39-92.79% yield. All compounds were prepared as 5% oil in water emulsion and measured its SPF and UVA/UVB values using an SPF-290S analyzer. The SPF values (n = 6) of the piperate derivatives were 2.68 +/- 0.17, 8.89 +/- 0.46, 6.86 +/- 0.91, 16.37 +/- 1.8, and 9.68 +/- 1.71. The UVA/UVB ratios of all compounds ranged from 0.860 to 0.967. Cytotoxicity of isopropyl piperate was evaluated using human skin fibroblast cells and the IC50 was equal to 120.2 muM. DISCUSSION AND CONCLUSION: From the results, isopropyl piperate is an outstanding compound that can be developed into a UV protection agent.
Active ingredients from natural botanicals in the treatment of obesity.[Pubmed:25417736]
Obes Rev. 2014 Dec;15(12):957-67.
Obesity is considered as a chronic disease that can induce a series of comorbidities and complications. Chinese medicine has long clinical experiences in the treatment of obesity. This review summarizes the natural products from traditional Chinese medicine (TCM) that are reported to have anti-obesity effects in the past two decades. Botanic TCM comprises 90% of total Chinese crude drugs, and generally contains various active ingredients, in which the effective anti-obesity ingredients identified can be divided into saponins, polysaccharides, alkaloids, polyphenols and others. Astragaloside IV, glycyrrhizin, macrostemonoside A, berberine, betaine, capsaicin, matrine, Methyl piperate, piperine, rutaecarpine, asimilobine, epigallocatechingallate, magnolol, resveratrol, soybean-isoflavone, alpha-linolenic acid, emodin, geniposide, phillyrin, salidroside and ursolic acid are specified in this review, and their sources, models, efficacy are described. It is concluded that the mechanisms of these components for the treatment of obesity include: (i) suppression of appetite, increase of satiety, reduction of energy intake; (ii) reduction in the digestion and absorption of exogenous lipid; (iii) attenuation of the synthesis of endogenous lipid; (iv) promotion of the oxidation and expenditure of lipid and (v) improvement of lipid metabolism disorder. Authors believe that the effective compounds from TCM will provide an alternative and hopeful way for the treatment of obesity.
[Chemical constituents from air-dried Piper longum].[Pubmed:19685743]
Zhongguo Zhong Yao Za Zhi. 2009 May;34(9):1101-3.
OBJECTIVE: To study the chemical constituents of Piper longum. METHOD: The whole plant of air-dried P. longum. was extracted with 95% EtOH. The EtOH extract was suspended in H2O and extracted with petroleum ether, CHC13 and n-BuOH, successively. The compounds were isolated and purified by column chromatography from the CHCl3 fraction, and identified based on spectral analyses (MS,1H-NMR, 13C-NMR). RESULT: Threeteen compounds were isolated from P. longum, and were characterized as 1-(3',4'-methylenedioxyphenyl)-1E-tetradecene (1), 3-(3', 4'-methylenedioxophenyl)-propenal (2), piperoic acid (3), 3',4'-di-hydroxy-biabola-1,10-diene (4), eudesm-4(15)-ene-1beta, 6alpha-diol (5), 7-epi- eudesm-4( 15)-ene-1beta, 6beta-diol (6), guineesine (7), piperine (8), pipericide (9), 2E, 4E-dienamide (10), (2E, 4E, 8E) -N-isobutylhenicosa-2,4,8-trienamide (11), piperlonguminine (12), Methyl piperate (13), CONCLUSION: Compounds 1-6 were obtained from P. longum for the first time.
Antioxidant effects of a Rhodobryum roseum extract and its active components in isoproterenol-induced myocardial injury in rats and cardiac myocytes against oxidative stress-triggered damage.[Pubmed:19216232]
Pharmazie. 2009 Jan;64(1):53-7.
The aim of this study was to investigate (1) whether Rhodobryum roseum, a traditional Chinese medicine used to treat cardiac disease, can protect myocardium damage due to isoproterenol-induced injury, (2) whether the cardioprotective effect of the R. roseum extract is related to its antioxidant activity, and (3) to identify the active components of R. roseum using the oxidant-mediated injury in cardiomyocytes. R. roseum was extracted with 95% EtOH (RE-95), 50% EtOH (RE-50) and water (Re-H2O) and the rats were treated orally for 11 days at doses of 250 mg and 63 mg/kg respectively after cardiac necrosis was induced by administering ISO subcutaneously at a dose of 85 mg/kg body weight. Levels of marker enzymes (LDH, GOT and CK) were assessed in serum whilst the antioxidant parameters, superoxide dismutase (SOD), and malondialdehde (MDA) were assayed in heart homogenate. Significant myocardial necrosis, depletion of endogenous antioxidants and an increase in serum levels of marker enzymes was observed in ISO-treated animals when compared with the normal animals. The RE-50 elicited a significant cardioprotective effect by lowering the levels of serum marker enzymes, lipid peroxidation (MDA). To extend this work, we sought to investigate the antioxidant effects of the components of R. roseum, using the neonatal rat cardiomyocytes model of H2O2-induced oxidant injury. Among the four major components, piperine and Methyl piperate significantly reduced the medium level of CK and LDH at a variety of dosages. Moreover, piperine and Methyl piperate significantly attenuated 2',7'-dichlorofluorescein (DCF) fluorescence by 63.9% and 52.6%, respectively. The present findings demonstrate that the cardioprotective effects of extracted R. roseum in ISO-induced oxidative damage may be due to an augmentation of the endogenous antioxidants and inhibition of lipid peroxidation of the membranes. Moreover, its components piperine and Methyl piperate exert significant protectective effects on cardiac myocytes.
Chemical constituents and bioactivity of Piper sarmentosum.[Pubmed:15234750]
J Ethnopharmacol. 2004 Aug;93(2-3):173-6.
Eight amides, pellitorine (1), guineensine (2), brachystamide B (3), sarmentine (4), brachyamide B (5), 1-piperettyl pyrrolidine (6), 3',4',5'-trimethoxycinnamoyl pyrrolidine (7) and sarmentosine (8), two lignans, (+)-asarinin (9) and sesamin (10), and four other compounds, 1-(3,4-methylenedioxyphenyl)-1E-tetradecene (11), Methyl piperate (12) and a mixture of beta-sitosterol (13) and stigmasterol (14), were isolated from the fruits of Piper sarmentosum (Piperaceae). This is the first reported isolation of compounds 2, 3, 5, 6, 7, 9, 10 and 12 from this plant species. Their structures were established from spectral data. These compounds were evaluated in antituberculosis and antiplasmodial tests. The results showed that compounds 4 and 6 exhibited both activities while compounds 1, 2, 5, 8 and 11 showed only antituberculosis activity. This is the first report of the antituberculosis and antiplasmodial activities for these compounds.
New amides and gastroprotective constituents from the fruit of Piper chaba.[Pubmed:14994194]
Planta Med. 2004 Feb;70(2):152-9.
The 80 % aqueous acetone extract from the fruit of Piper chaba was found to show protective effects on ethanol- and indomethacin-induced gastric lesions in rats. From the aqueous acetone extract, four new amides named piperchabamides A ( 1), B ( 2), C ( 3), and D ( 4) were isolated, and their structures were determined on the basis of chemical and physicochemical evidence. In addition, the gastroprotective effects of the principal constituents, piperine ( 5), piperanine ( 6), pipernonaline ( 7), dehydropipernonaline ( 8), piperlonguminine ( 9), retrofractamide B ( 10), guineensine ( 11), N-isobutyl-(2 E,4 E)-octadecadienamide ( 12), N-isobutyl-(2 E,4 E,14 Z)-eicosatrienamide ( 13), and Methyl piperate ( 14), were examined. As a result, compounds 5 - 10 and 12 - 14 significantly inhibited ethanol-induced gastric lesions at a dose of 25 mg/kg, p. o., while 5, 7, 8, 10, 12, and 13 also significantly inhibited indomethacin-induced gastric lesions at the same dose.
A New Linalool Derivative and Other Constituents from Piper ribesoides.[Pubmed:17262340]
Planta Med. 1989 Apr;55(2):193-4.
Extraction of PIPER RIBESOIDES Wall, furnished the new linalool derivative (+)-3,7-dimethyl-3-hydroxy-4-( P-coumaryloxy)-1,6-octadiene, the lignans (-)-hinokinin and (-)-cubebin, N-isobutyl-2 E,4 E-deca-2,4-dienamide, Methyl piperate, methyl 2 E,4 E,6 E-7-phenyl-2,4,6-heptatrienoate, palmitic acid, stearic acid, and beta-sitosterol.


