PBP 10Selective FPR2 antagonist; cell permeable CAS# 794466-43-6 |
- AM630
Catalog No.:BCC1353
CAS No.:164178-33-0
- Nepicastat
Catalog No.:BCC1795
CAS No.:173997-05-2
- JWH 073
Catalog No.:BCC1674
CAS No.:208987-48-8
- CP-945598 HCl
Catalog No.:BCC1082
CAS No.:686347-12-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 794466-43-6 | SDF | Download SDF |
| PubChem ID | 90488957 | Appearance | Powder |
| Formula | C84H126N24O15 | M.Wt | 1712.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 2 mg/ml in water | ||
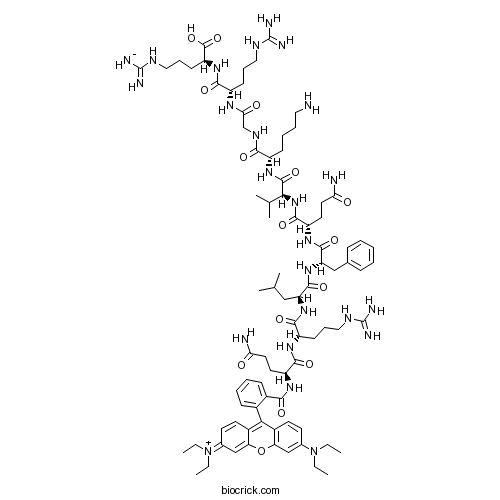
| Sequence | QRLFQVKGRR (Modifications: Gln-1 = Rhodamine-B) | ||
| SMILES | CCN(CC)C1=CC2=C(C=C1)C(=C3C=CC(=[N+](CC)CC)C=C3O2)C4=CC=CC=C4C(=O)NC(CCC(=O)N)C(=O)NC(CCCNC(=N)N)C(=O)NC(CC(C)C)C(=O)NC(CC5=CC=CC=C5)C(=O)NC(CCC(=O)N)C(=O)NC(C(C)C)C(=O)NC(CCCCN)C(=O)NCC(=O)NC(CCCNC(=N)N)C(=O)NC(CCCNC(=N)[NH-])C(=O)O | ||
| Standard InChIKey | OIOWMSSANHIUTR-NVWDZAHRSA-N | ||
| Standard InChI | InChI=1S/C84H126N24O15/c1-9-107(10-2)51-31-33-55-65(45-51)123-66-46-52(108(11-3)12-4)32-34-56(66)70(55)53-25-16-17-26-54(53)72(112)99-60(35-37-67(86)109)76(116)100-59(29-21-41-95-83(90)91)75(115)104-63(43-48(5)6)78(118)105-64(44-50-23-14-13-15-24-50)79(119)101-61(36-38-68(87)110)77(117)106-71(49(7)8)80(120)102-57(27-18-19-39-85)73(113)97-47-69(111)98-58(28-20-40-94-82(88)89)74(114)103-62(81(121)122)30-22-42-96-84(92)93/h13-17,23-26,31-34,45-46,48-49,57-64,71H,9-12,18-22,27-30,35-44,47,85H2,1-8H3,(H26-,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,109,110,111,112,113,114,115,116,117,118,119,120,121,122)/t57-,58-,59-,60-,61-,62-,63-,64-,71-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective formyl peptide receptor 2 (FPR2) antagonist; cell permeable. Selectively inhibits FPR2-mediated NADPH oxidase activity but has no effect on FPR1 signaling in neutrophils. Displays PIP2 binding activity in vitro and blocks cell motility. Also exhibits antiviral activity against influenza viruses via inhibition of viral-induced ERK activation. |

PBP 10 Dilution Calculator

PBP 10 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 360A
Catalog No.:BCC1307
CAS No.:794458-56-3
- Bruceantinoside A
Catalog No.:BCN7622
CAS No.:79439-85-3
- Yadanzioside P
Catalog No.:BCN6711
CAS No.:79439-84-2
- 3alpha-Cinnamoyloxypterokaurene L3
Catalog No.:BCN4575
CAS No.:79406-13-6
- 3alpha-Angeloyloxypterokaurene L3
Catalog No.:BCN4576
CAS No.:79406-11-4
- ent-3beta-Cinnamoyloxykaur-16-en-19-oic acid
Catalog No.:BCN1349
CAS No.:79406-10-3
- ent-3Beta-Tigloyloxykaur-16-en-19-oic acid
Catalog No.:BCN1350
CAS No.:79406-09-0
- BIBU 1361 dihydrochloride
Catalog No.:BCC7356
CAS No.:793726-84-8
- AAL Toxin TA1
Catalog No.:BCN1733
CAS No.:79367-52-5
- AAL Toxin TA2
Catalog No.:BCN1738
CAS No.:79367-51-4
- Cefixime
Catalog No.:BCC8907
CAS No.:79350-37-1
- H-D-Glu-OBzl
Catalog No.:BCC2937
CAS No.:79338-14-0
- Vernakalant
Catalog No.:BCC2036
CAS No.:794466-70-9
- Nicaraven
Catalog No.:BCC4684
CAS No.:79455-30-4
- Eleutheroside D
Catalog No.:BCN5336
CAS No.:79484-75-6
- 9-Oxo-10,11-dehydroageraphorone
Catalog No.:BCN4333
CAS No.:79491-71-7
- Glaucocalyxin A
Catalog No.:BCN2353
CAS No.:79498-31-0
- Norketamine hydrochloride
Catalog No.:BCC5859
CAS No.:79499-59-5
- Stelleranol
Catalog No.:BCN8014
CAS No.:795308-62-2
- 20-HETE
Catalog No.:BCC1301
CAS No.:79551-86-3
- L-165041
Catalog No.:BCC1687
CAS No.:79558-09-1
- 5-Ethoxychelerthrine
Catalog No.:BCC8105
CAS No.:79559-55-0
- 1,7-Diphenyl-4-hepten-3-one
Catalog No.:BCN3592
CAS No.:79559-59-4
- Sertraline HCl
Catalog No.:BCC5059
CAS No.:79559-97-0
Interaction of the gelsolin-derived antibacterial PBP 10 peptide with lipid bilayers and cell membranes.[Pubmed:16940084]
Antimicrob Agents Chemother. 2006 Sep;50(9):2932-40.
PBP 10, an antibacterial, cell membrane-permeant rhodamine B-conjugated peptide derived from the polyphosphoinositide binding site of gelsolin, interacts selectively with both lipopolysaccharides (LPS) and lipoteichoic acid (LTA), the distinct components of gram-negative and gram-positive bacteria, respectively. Isolated LPS and LTA decrease the antimicrobial activities of PBP 10, as well as other antimicrobial peptides, such as cathelicidin-LL37 (LL37) and mellitin. In an effort to elucidate the mechanism of bacterial killing by PBP 10, we compared its effects on artificial lipid bilayers and eukaryotic cell membranes with the actions of the mellitin, magainin II, and LL37 peptides. This study reveals that pore formation is unlikely to be involved in PBP 10-mediated membrane destabilization. We also investigated the effects of these peptides on platelets and red blood cells (RBCs). Comparison of these antimicrobial peptides shows that only mellitin has a toxic effect on platelets and RBCs in a concentration range concomitant with its bactericidal activity. The hemolytic activities of the PBP 10 and LL37 peptides significantly increase when RBCs are osmotically swollen in hypotonic solution, indicating that these antibacterial peptides may take advantage of the more extended form of bacterial membranes in exerting their killing activities. Additionally, we found that LL37 hemolytic activity was much higher when RBCs were induced to expose phosphatidylserine to the external leaflet of their plasma membranes. This finding suggests that asymmetrical distribution of phospholipids in the external membranes of eukaryotic cells may represent an important factor in determining the specificity of antibacterial peptides for targeting bacteria rather than eukaryotic cells.
Structural characterization and inhibitory profile of formyl peptide receptor 2 selective peptides descending from a PIP2-binding domain of gelsolin.[Pubmed:22706076]
J Immunol. 2012 Jul 15;189(2):629-37.
The neutrophil formyl peptide receptors, FPR1 and FPR2, play critical roles for inflammatory reactions, and receptor-specific antagonists/inhibitors can possibly be used to facilitate the resolution of pathological inflammatory reactions. A 10-aa-long rhodamine-linked and membrane-permeable peptide inhibitor (PBP10) has such a potential. This FPR2 selective inhibitor adopts a phosphatidylinositol 4,5-bisphosphate-binding sequence in the cytoskeletal protein gelsolin. A core peptide, RhB-QRLFQV, is identified that displays inhibitory effects as potent as the full-length molecule. The phosphatidylinositol 4,5-bisphosphate-binding capacity of PBP10 was not in its own sufficient for inhibition. A receptor in which the presumed cytoplasmic signaling C-terminal tail of FPR2 was replaced with that of FPR1 retained the PBP10 sensitivity, suggesting that the tail of FPR2 was not on its own critical for inhibition. This gains support from the fact that the effect of cell-penetrating lipopeptide (a pepducin), suggested to act primarily through the third intracellular loop of FPR2, was significantly inhibited by PBP10. The third intracellular loops of FPR1 and FPR2 differ in only two amino acids, but an FPR2 mutant in which these two amino acids were replaced by those present in FPR1 retained the PBP10 sensitivity. In summary, we conclude that the inhibitory activity on neutrophil function of PBP10 is preserved in the core sequence RhB-QRLFQV and that neither the third intracellular loop of FPR2 nor the cytoplasmic tail of the receptor alone is responsible for the specific inhibition.
Cell permeant polyphosphoinositide-binding peptides that block cell motility and actin assembly.[Pubmed:11533030]
J Biol Chem. 2001 Nov 16;276(46):43390-9.
Polyphosphoinositides (PPIs) affect the localization and activities of many cellular constituents, including actin-modulating proteins. Several classes of polypeptide sequences, including pleckstrin homology domains, FYVE domains, and short linear sequences containing predominantly hydrophobic and cationic residues account for phosphoinositide binding by most such proteins. We report that a ten-residue peptide derived from the phosphatidylinositol 4,5-bisphosphate (PIP(2)) binding region in segment 2 of gelsolin, when coupled to rhodamine B has potent PIP(2) binding activity in vitro; crosses the cell membrane of fibroblasts, platelets, melanoma cells, and neutrophils by a process not involving endocytosis; and blocks cell motility. This peptide derivative transiently disassembles actin filament structures in GFP-actin-expressing NIH3T3 fibroblasts and prevents thrombin- or chemotactic peptide-stimulated actin assembly in platelets and neutrophils, respectively, but does not block the initial [Ca(2+)] increase caused by these agonists. The blockage of actin assembly and motility is transient, and cells recover motility within an hour after their immobilization by 5-20 microm peptide. This class of reagents confirms the critical relation between inositol lipids and cytoskeletal structure and may be useful to probe the location and function of polyphosphoinositides in vivo.


