Phomaligol ACAS# 152204-32-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 152204-32-5 | SDF | Download SDF |
| PubChem ID | 15265875 | Appearance | Oil |
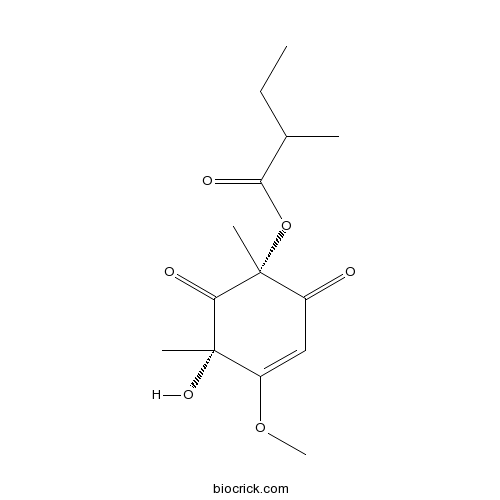
| Formula | C14H20O6 | M.Wt | 284.3 |
| Type of Compound | Other NPs | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1R,5S)-5-hydroxy-4-methoxy-1,5-dimethyl-2,6-dioxocyclohex-3-en-1-yl] 2-methylbutanoate | ||
| SMILES | CCC(C)C(=O)OC1(C(=O)C=C(C(C1=O)(C)O)OC)C | ||
| Standard InChIKey | DWJRXSZPSOQYDZ-AZGGKPGBSA-N | ||
| Standard InChI | InChI=1S/C14H20O6/c1-6-8(2)11(16)20-14(4)9(15)7-10(19-5)13(3,18)12(14)17/h7-8,18H,6H2,1-5H3/t8?,13-,14+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Phomaligol A Dilution Calculator

Phomaligol A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5174 mL | 17.5871 mL | 35.1741 mL | 70.3482 mL | 87.9353 mL |
| 5 mM | 0.7035 mL | 3.5174 mL | 7.0348 mL | 14.0696 mL | 17.5871 mL |
| 10 mM | 0.3517 mL | 1.7587 mL | 3.5174 mL | 7.0348 mL | 8.7935 mL |
| 50 mM | 0.0703 mL | 0.3517 mL | 0.7035 mL | 1.407 mL | 1.7587 mL |
| 100 mM | 0.0352 mL | 0.1759 mL | 0.3517 mL | 0.7035 mL | 0.8794 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Polyhydroxybutyrate
Catalog No.:BCN9665
CAS No.:26744-04-7
- (+)-Hannokinol
Catalog No.:BCN9664
CAS No.:408324-76-5
- (±)-Magnolin
Catalog No.:BCN9663
CAS No.:1275595-33-9
- (±)-Lariciresinol
Catalog No.:BCN9662
CAS No.:105367-81-5
- Trolline
Catalog No.:BCN9661
CAS No.:1021950-79-7
- Plucheoside B aglycone
Catalog No.:BCN9660
CAS No.:393862-19-6
- benzyl hydrogen (3-(((2R,3S)-2-((R)-1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)-3-(4-fluorophenyl)morpholino)methyl)-5-imino-4,5-dihydro-1H-1,2,4-triazol-1-yl)phosphonate
Catalog No.:BCN9659
CAS No.:889852-02-2
- Dexamethasone 21-phosphate disodium salt
Catalog No.:BCN9658
CAS No.:2392-39-4
- Ethyl 3-(4-(methylamino)-3-nitro-N-(pyridin-2-yl)benzamido)propanoate
Catalog No.:BCN9657
CAS No.:429659-01-8
- Orange IV
Catalog No.:BCN9656
CAS No.:554-73-4
- Saccharin sodium
Catalog No.:BCN9655
CAS No.:128-44-9
- Acid Orange 7
Catalog No.:BCN9654
CAS No.:633-96-5
- Euglobal Ia2
Catalog No.:BCN9667
CAS No.:77794-63-9
- 4-Chloropinselin
Catalog No.:BCN9668
CAS No.:104022-83-5
- Herbaridine B
Catalog No.:BCN9669
CAS No.:1151511-05-5
- 18-Hydroxytritriacontan-16-one
Catalog No.:BCN9670
CAS No.:97191-42-9
- (2Z,6Z,10E,14E,18E)-Farnesylfarnesol
Catalog No.:BCN9671
CAS No.:90695-03-7
- 5-O-Ethylcleroindicin D
Catalog No.:BCN9672
CAS No.:488138-32-5
- 3-Hydroxychimaphilin
Catalog No.:BCN9673
CAS No.:33253-99-5
- Cerebroside D
Catalog No.:BCN9674
CAS No.:113773-89-0
- Dehydroacerogenin C
Catalog No.:BCN9675
CAS No.:152041-27-5
- 8-Methoxyfissistigine C
Catalog No.:BCN9676
CAS No.:20824-18-4
- 10-Epiteuclatriol
Catalog No.:BCN9677
CAS No.:151563-36-9
- Jaslanceoside B
Catalog No.:BCN9678
CAS No.:188300-82-5
A new cyclohexenone from the tin mine tailings-derived fungus Aspergillus flavus YIM DT 10012.[Pubmed:29376405]
Nat Prod Res. 2019 Jan;33(1):113-116.
A new cyclohexenone, named phomaligol D (1), together with two known compounds, kojic acid (2) and Phomaligol A (3) were isolated from the tin mine tailings-derived fungus Aspergillus flavus YIM DT 10012. Their structures were elucidated by detailed analysis of spectroscopic data.
Flavusides A and B, antibacterial cerebrosides from the marine-derived fungus Aspergillus flavus.[Pubmed:21881265]
Chem Pharm Bull (Tokyo). 2011;59(9):1174-7.
Flavusides A (1) and B (2), two new antibacterial cerebroside derivatives, and the previously described Phomaligol A (3), kojic acid (4), methyl kojic acid (5), and dimethyl kojic acid (6) have been isolated from the extract of a marine isolate of the fungus Aspergillus flavus. The structure and absolute stereochemistry of two cerebrosides were assigned on the basis of NMR and Tandem FAB-MS/MS experiments. Compounds 1, 2, and 3 exhibited a mild antibacterial activity against Staphylococcus aureus, methicillin-resistant S. aureus, and multidrug-resistant S. aureus. The minimum inhibitory concentration (MIC) values for each strain are as follows: compounds 1 and 2 showed 15.6 mug/ml for S. aureus and 31.2 mug/ml for methicillin-resistant S. aureus and multidrug-resistant S. aureus, and compound 3 exhibited 31.2 mug/ml for S. aureus and methicillin-resistant S. aureus and 62.5 mug/ml for multidrug-resistant S. aureus.
Gamma-pyrone derivatives, kojic acid methyl ethers from a marine-derived fungus Alternaria [correction of Altenaria] sp.[Pubmed:12934644]
Arch Pharm Res. 2003 Jul;26(7):532-4.
Kojic acid dimethyl ether (1), and the known kojic acid monomethyl ether (2), kojic acid (3) and Phomaligol A (4) have been isolated from the organic extract of the broth of the marine-derived fungus Alternaria sp. collected from the surface of the marine green alga Ulva pertusa. The structures were assigned on the basis of comprehensive spectroscopic analyses. Each isolate was tested for its tyrosinase inhibitory activity. Kojic acid (3) was found to have significant tyrosinase inhibitory activity, but compounds 1, 2, and 4 were found to be inactive.


