Acid Orange 7CAS# 633-96-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 633-96-5 | SDF | Download SDF |
| PubChem ID | 135442941 | Appearance | Red powder |
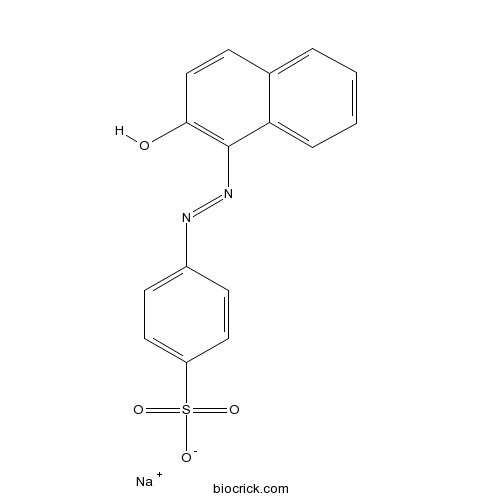
| Formula | C16H11N2NaO4S | M.Wt | 350.3 |
| Type of Compound | Impurities | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | sodium;4-[(2-hydroxynaphthalen-1-yl)diazenyl]benzenesulfonate | ||
| SMILES | C1=CC=C2C(=C1)C=CC(=C2N=NC3=CC=C(C=C3)S(=O)(=O)[O-])O.[Na+] | ||
| Standard InChIKey | CQPFMGBJSMSXLP-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C16H12N2O4S.Na/c19-15-10-5-11-3-1-2-4-14(11)16(15)18-17-12-6-8-13(9-7-12)23(20,21)22;/h1-10,19H,(H,20,21,22);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Acid Orange 7 Dilution Calculator

Acid Orange 7 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8547 mL | 14.2735 mL | 28.547 mL | 57.0939 mL | 71.3674 mL |
| 5 mM | 0.5709 mL | 2.8547 mL | 5.7094 mL | 11.4188 mL | 14.2735 mL |
| 10 mM | 0.2855 mL | 1.4273 mL | 2.8547 mL | 5.7094 mL | 7.1367 mL |
| 50 mM | 0.0571 mL | 0.2855 mL | 0.5709 mL | 1.1419 mL | 1.4273 mL |
| 100 mM | 0.0285 mL | 0.1427 mL | 0.2855 mL | 0.5709 mL | 0.7137 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 17-Ethylendioxyandrosta-1,4-dien-3-one
Catalog No.:BCN9653
CAS No.:2398-63-2
- 1-Benzyl D-aspartate
Catalog No.:BCN9652
CAS No.:79337-40-9
- N,N'-Bis(2-hydroxyethyl)ethylenediamine
Catalog No.:BCN9651
CAS No.:4439-20-7
- Bisdemethoxycucurmin
Catalog No.:BCN9650
CAS No.:24939-16-0
- Benzalacetone
Catalog No.:BCN9649
CAS No.:122-57-6
- 1,2,3,4-Tetrahydro-1-acetylquinoline
Catalog No.:BCN9648
CAS No.:4169-19-1
- tert-Butyl 6-[(1E)-2-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetate
Catalog No.:BCN9647
CAS No.:289042-12-2
- 5-[2-[[(4-Methoxyphenyl)diphenylmethyl]amino]-6-(phenylmethoxy)-9H-purin-9-yl]-3-(phenylmethoxy)-2-[(phenylmethoxy)methyl]cyclopentanol
Catalog No.:BCN9646
CAS No.:142217-78-5
- Teriflunomide
Catalog No.:BCN9645
CAS No.:163451-81-8
- 2-[(1S,2S)-1-Ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-3H-1,2,4-triazol-3-one
Catalog No.:BCN9644
CAS No.:184177-83-1
- Toluene-4-sulfonic acid 5-(2,4-difluorophenyl)-5-(1H-1,2,4-triazol-1-yl)methyltetrahydrofuran-3-ylmethyl ester
Catalog No.:BCN9643
CAS No.:149809-43-8
- 3-[[[2-[[(4-Cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]pyridin-2-ylamino]propionic acid ethyl ester
Catalog No.:BCN9642
CAS No.:211915-84-3
- Saccharin sodium
Catalog No.:BCN9655
CAS No.:128-44-9
- Orange IV
Catalog No.:BCN9656
CAS No.:554-73-4
- Ethyl 3-(4-(methylamino)-3-nitro-N-(pyridin-2-yl)benzamido)propanoate
Catalog No.:BCN9657
CAS No.:429659-01-8
- Dexamethasone 21-phosphate disodium salt
Catalog No.:BCN9658
CAS No.:2392-39-4
- benzyl hydrogen (3-(((2R,3S)-2-((R)-1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)-3-(4-fluorophenyl)morpholino)methyl)-5-imino-4,5-dihydro-1H-1,2,4-triazol-1-yl)phosphonate
Catalog No.:BCN9659
CAS No.:889852-02-2
- Plucheoside B aglycone
Catalog No.:BCN9660
CAS No.:393862-19-6
- Trolline
Catalog No.:BCN9661
CAS No.:1021950-79-7
- (±)-Lariciresinol
Catalog No.:BCN9662
CAS No.:105367-81-5
- (±)-Magnolin
Catalog No.:BCN9663
CAS No.:1275595-33-9
- (+)-Hannokinol
Catalog No.:BCN9664
CAS No.:408324-76-5
- Polyhydroxybutyrate
Catalog No.:BCN9665
CAS No.:26744-04-7
- Phomaligol A
Catalog No.:BCN9666
CAS No.:152204-32-5
[Degradation of AO7 with Magnetic Fe3O4-CuO Heterogeneous Catalyzed Sodium Percarbonate System].[Pubmed:32608680]
Huan Jing Ke Xue. 2020 Apr 8;41(4):1734-1742.
Magnetically recyclable Fe3O4-CuO was synthesized by a one-step hydrothermal method and characterized by scanning electron microscopy coupled with energy dispersive spectrometer (SEM-EDS) and X-ray diffraction (XRD). The degradation of azo dye Acid Orange 7 (AO7) by percarbonate (SPC) activated with Fe3O4-CuO was studied. The effects of Fe3O4-CuO catalyst loading, SPC concentration, pH value, and common chloride ions on AO7 degradation in the Fe3O4-CuO/SPC system were evaluated. The main reaction mechanism of AO7 degradation was analyzed. The results show that Fe3O4-CuO could effectively activate SPC to degrade AO7 and the reaction was accelerated with the increase of Fe3O4-CuO dosage. The increase of SPC dosage was favorable for the degradation of AO7, but excessive SPC dosage inhibited the degradation of AO7. Common ions (e.g., Cl(-)) in dye wastewater could promote the degradation of AO7, and the degradation rate increased with increasing concentration of Cl(-). The reaction mainly occurred on the surface of the catalyst, and.OH was identified as the main active species for the degradation of AO7. The catalyst Fe3O4-CuO showed excellent stability owing to the high catalytic activity remaining after 4 cycles of repeated use. The Fe3O4-CuO/SPC system achieved a high mineralization rate in the process of decolorization of AO7.
The removal of azo dye from aqueous solution by oxidation with peroxydisulfate in the presence of granular activated carbon: Performance, mechanism and reusability.[Pubmed:32593002]
Chemosphere. 2020 Jun 17;259:127400.
Granular activated carbon (GAC) was used as catalyst for the activation of peroxydisulfate (PDS) to decolorize and degrade Acid Orange 7 (AO7) in water. EPR spectra and radical quencher experiments were employed to identify the active species for AO7 oxidation in the PDS/GAC system. Linear sweep voltammetry (LSV) and chronoamperometry test were carried out to identify the contribution of nonradical mechanism for AO7 decay. The investigation of crucial operational parameters on the decolorization indicated 100 mg/L AO7 can be almost totally decolorized in a broad range of pH. Common inorganic anions adversely affect the AO7 decolorization process and the inhibition was in the order of: HCO3(-) > H2PO4(-) > SO4(2-) > Cl(-) > NO3(-). UV-vis spectra showed the destruction of the aromatic moiety of AO7 molecule during the oxidation reaction of the PDS/GAC system. The transformation of nitrogen related to the azo bond in AO7 molecule in this system was observed by monitoring the released N-containing inorganic ions. Recycle experiments showed GAC cannot be reused directly but its catalytic ability can be restored by using electrochemical method.
Response surface methodology and artificial neural network for remediation of acid orange 7 using TiO2-P25: optimization and modeling approach.[Pubmed:32557068]
Environ Sci Pollut Res Int. 2020 Jun 15. pii: 10.1007/s11356-020-09674-4.
The primary responsibility for continuously discharging toxic organic pollutants into water bodies and open environments is the increase in industrial and agricultural activities. Developing economical and suitable methods to continuously remove organic pollutants from wastewater is highly essential. The aim of the present research was to apply response surface methodology (RSM) and artificial neural networks (ANNs) for optimization and modeling of photocatalytic degradation of Acid Orange 7 (AO7) by commercial TiO2-P25 nanoparticles (TNPs). Dose of TNPs, pH, and AO7 concentration were selected as investigated parameters. RSM results reveal the reflective rate of AO7 removal of ~ 94.974% was obtained at pH 7.599, TNP dose of 0.748 g/L, and AO7 concentration of 28.483 mg/L. The resulting quadratic model is satisfactory with the highest coefficient of determination (R(2)) between the predicted and experimental data (R(2) = 0.98 and adjusted R(2) = 0.954). On the other hand, ANNs were successfully employed for modeling of AO7 degradation process. The proposed ANN model was absolutely fitted with experimental results producing the highest R(2). Furthermore, root mean square error (RMSE), mean average deviation (MAD), absolute average relative error (AARE), and mean square error (MSE) were examined more to compare the predictive capabilities of ANN and RSM models. The experimental data was well fitted into pseudo-first-order and pseudo-second-order kinetics with more accuracy. Thermodynamic parameters, namely enthalpy, entropy, Gibbs' free energy, and activation energy, were also evaluated to suggest the nature of the degradation process. The increase of temperature was analyzed to be more suitable for the fast removal of AO7 over TNPs. Graphical abstract.
Synthesis and characterization of magnetic biochar adsorbents for the removal of Cr(VI) and Acid orange 7 dye from aqueous solution.[Pubmed:32519109]
Environ Sci Pollut Res Int. 2020 Jun 10. pii: 10.1007/s11356-020-09275-1.
In this study, different types of magnetic biochar nanocomposites were synthesized using the co-precipitation method. Two biochar materials, namely, sewage sludge biochar and woodchips biochar, were prepared at two different temperatures, viz., 450 and 700 degrees C. These biochars were further modified with magnetic nanoparticles (Fe3O4). The modified biochar nanocomposites were characterized using field emission-scanning electron microscopy (FE-SEM), X-ray diffraction (XRD), transmission electron microscopy (TEM), Brunauer-Emmett-Teller (BET), SQUID analysis, X-ray photoelectron spectroscopy (XPS), and Fourier-transform infrared spectroscopy (FTIR). The potential of prepared adsorbents was examined for the removal of hexavalent chromium (Cr(VI)) and Acid Orange 7 (AO7) dye from water as a function of various parameters, namely, contact time, pH of solution, amount of adsorbents, and initial concentrations of adsorbates. Various kinetic and isotherm models were tested to discuss and interpret the adsorption mechanisms. The maximum adsorption capacities of modified biochars were found as 80.96 and 110.27 mg g(-1) for Cr(VI) and AO7, respectively. Magnetic biochars showed high pollutant removal efficiency after 5 cycles of adsorption/desorption. The results of this study revealed that the prepared adsorbents can be successfully used for multiple cycles to remove Cr(VI) and AO7 from water. Graphical Abstract.
Constructed wetland-microbial fuel cell for azo dyes degradation and energy recovery: Influence of molecular structure, kinetics, mechanisms and degradation pathways.[Pubmed:32325554]
Sci Total Environ. 2020 Jun 10;720:137370.
Complete degradation of azo dye has always been a challenge due to the refractory nature of azo dye. An innovative hybrid system, constructed wetland-microbial fuel cell (CW-MFC) was developed for simultaneous azo dye remediation and energy recovery. This study investigated the effect of circuit connection and the influence of azo dye molecular structures on the degradation rate of azo dye and bioelectricity generation. The closed circuit system exhibited higher chemical oxygen demand (COD) removal and decolourisation efficiencies compared to the open circuit system. The wastewater treatment performances of different operating systems were ranked in the decreasing order of CW-MFC (R1 planted-closed circuit) > MFC (R2 plant-free-closed circuit) > CW (R1 planted-open circuit) > bioreactor (R2 plant-free-open circuit). The highest decolourisation rate was achieved by Acid Red 18 (AR18), 96%, followed by Acid Orange 7 (AO7), 67% and Congo Red (CR), 60%. The voltage outputs of the three azo dyes were ranked in the decreasing order of AR18 > AO7 > CR. The results disclosed that the decolourisation performance was significantly influenced by the azo dye structure and the moieties at the proximity of azo bond; the naphthol type azo dye with a lower number of azo bond and more electron-withdrawing groups could cause azo bond to be more electrophilic and more reductive for decolourisation. Moreover, the degradation pathway of AR18, AO7 and CR were elucidated based on the respective dye intermediate products identified through UV-Vis spectrophotometry, high-performance liquid chromatography (HPLC), and gas chromatograph-mass spectrometer (GC-MS) analyses. The CW-MFC system demonstrated high capability of decolouring azo dyes at the anaerobic anodic region and further mineralising dye intermediates at the aerobic cathodic region to less harmful or non-toxic products.
Biochar-activated persulfate for organic contaminants removal: Efficiency, mechanisms and influencing factors.[Pubmed:32325258]
Ecotoxicol Environ Saf. 2020 Apr 20;198:110653.
Turning biomass into biochar as a multifunctional carbon-based material for water remediation has attracted much research attention. Sawdust and rice husk were selected as feedstock for biochar (BC) production, aiming to explore their performance as a catalyst to activate persulfate (PS) for degrading Acid Orange 7 (AO7). There was an excellent synergistic effect in the combined BC/PS system. Sawdust biochar (MX) showed a faster and more efficient performance for the AO7 degradation due to its abundant oxygen functional groups, compared to rice husk biochar (DK). In the BC/PS system, AO7 was well decolorized and mineralized. Based on the two-dimensional correlation analysis method, the azo conjugation structure and naphthalene ring of AO7 molecule changed first then benzene ring changed during the reaction. Moreover, AO7 decolorization efficiency increased with the increase of PS concentration and biochar dosage, and the deacrease of pH. Biochar deactivated after used twice. When the biochar reached its adsorption equilibrium of AO7, the AO7 could not be degraded in the BC/PS system. SO4(-) and OH participated in the reaction together and OH played the main role in activating PS to AO7 decolorization based on the radical scavengers experiment. All of results indicate using biochar to activate PS for degradation of AO7 contaminated water is a promising method.
Automatic microbial electro-Fenton system driven by transpiration for degradation of acid orange 7.[Pubmed:32302852]
Sci Total Environ. 2020 Jul 10;725:138508.
Microbial electro-Fenton system (MEFS) shows potential application for degradation of recalcitrant pollutants. In order to simplify the MEFS and adapt to the practical application situations, such as water, soil or sludge remediation, we developed an automatic MEFS (AMEFS) for degradation of a recalcitrant dye, Acid Orange 7. The AMEFS contained a microchannel-structured carbon decorated with iron oxides as electro-Fenton cathode. The AMEFS could be either two-electrode configuration that the microchannel-structured carbon connected with an additional bioanode by an external circuit, or single-electrode configuration that the microchannel-structured carbon served as both bioanode and cathode. Thanks to the microchannel structure of the carbon cathode, the AMEFS could be auto-driven by a process similar to the transpiration process of natural plants. The two-electrode AMEFS had higher degradation efficiency of Acid Orange 7 at lower external resistance, and achieved the highest degradation efficiency of 96% at the short-circuit condition. The single-electrode configuration simplified the setup of the AMEFS and possessed comparable performance with that of two-electrode configuration at short-circuit condition. Moreover, it could degrade high concentration Acid Orange 7 of up to 50 mg L(-1) and achieve a high degradation efficiency of over 93%. The AMEFS could be applied for soil and sludge remediation by direct insertion of the microchannel structured carbon into contaminated body.
Synthetic NiO catalyst-assisted peroxymonosulfate for degradation of benzoic acid from aqueous solution.[Pubmed:32281703]
Water Environ Res. 2020 Apr 13.
A heterogeneous NiO catalyst was prepared by a precipitation process using nickel nitrate with oxalic acid and tested for heterogeneous oxidation of benzoic acid (BA) in the presence of peroxymonosulfate (PMS). It was found that the synthetic NiO is highly effective in heterogeneous activation of PMS to produce sulfate radicals ( SO 4 . - ) and hydroxyl radicals ((.) OH), and also presents stable performance in the heterogeneous activation of PMS for BA degradation. Physicochemical properties of the NiO catalyst were characterized by several techniques, such as thermogravimetric analysis, Brunauer-Emmett-Teller, Fourier transform infrared spectroscopy, X-ray diffraction, X-ray photoelectron spectroscopy, scanning electron microscopy, and transmission electron microscopy. It was found that NiO and NiOOH were formed on the synthetic NiO catalyst and were stably distributed on the catalyst surface. Nearly 95% decomposition could be achieved in 30 min at the conditions of 500 ml 20 muM BA solution, 0.25 g catalyst, and [PMS]:[BA] = 30:1. The heterogeneous reactions, the effects of PMS concentration, and catalyst dosage on the BA degradation were investigated. The heterogeneous BA degradation reactions followed first-order kinetics. Additionally, quenching experiments proved that the dominant radical in the solution was (.) OH. The experiments results also showed that this approach is effective for the degradation of many other pollutants (such as tetracycline hydrochloride, 2, 4-dichlorophenol, Acid Orange 7, rhodamine B, and methyl red). PRACTITIONER POINTS: A novel NiO material was fabricated for degradation of benzoic acid. The synthetic NiO catalyst comprised active NiO and NiOOH. The main radical for benzoic acid removal rate was (.) OH. A plausible mechanism for catalyzed degradation of the benzoic acid was proposed.
Selective adsorption of organic pigments on inorganically modified mesoporous biochar and its mechanism based on molecular structure.[Pubmed:32268260]
J Colloid Interface Sci. 2020 Aug 1;573:21-30.
The treatment of organic pigments has gained significant attention worldwide owing to the large amounts of pollutants emitted during the process. The main purpose of this study is to prepare an environmentally friendly, low-cost adsorbent with high efficiency with selective adsorption for water purification. An inorganically modified mesoporous biochar derived from sorghum straw was synthesized in one step, and three typical organic pigments including methyl blue (MB), Acid Orange 7 (AO7), and alizarin red (AR) were selected as cationic, azo, and anionic pigments, respectively. The characterization results demonstrated that Fe3O4 particles successfully attached to the surface of biochar after modification. Although the enhanced adsorption behaviors of the three pigments on the modified biochar were described effectively by the Langmuir isotherm, the as-prepared materials showed a better selective adsorption effect on the cationic pigments. Moreover, the adsorption processes of all targeted pigments were endothermic and governed by entropy. Smaller molecular dimensions, lighter molecular weight, and the low molecular electrostatic potential of MB are responsible for its selective adsorption; however, the enhanced adsorption affinity is attributed to the hydrogen bond and n-pi interaction with the benzene ring of the pigment molecules.
Synthesis of Spinel Ferrite MFe2O4 (M = Co, Cu, Mn, and Zn) for Persulfate Activation to Remove Aqueous Organics: Effects of M-Site Metal and Synthetic Method.[Pubmed:32266209]
Front Chem. 2020 Mar 24;8:177.
Metal species and synthetic method determine the characteristics of spinel ferrite MFe2O4. Herein, a series of MFe2O4 (M = Co, Cu, Mn, Zn) were synthesized to investigate the effect of M-site metal on persulfate activation for the removal of organics from aqueous solution. Results showed that M-site metal of MFe2O4 significantly influenced the catalytic persulfate oxidation of organics. The efficiency of the removal of organics using different MFe2O4 + persulfate systems followed the order of CuFe2O4 > CoFe2O4 > MnFe2O4 > ZnFe2O4. Temperature-programmed oxidation and cyclic voltammetry analyses indicated that M-site metal affected the catalyst reducibility, reversibility of M(2+)/M(3+) redox couple, and electron transfer, and the strengths of these capacities were consistent with the catalytic performance. Besides, it was found that surface hydroxyl group was not the main factor affecting the reactivity of MFe2O4 in persulfate solution. Moreover, synthetic methods (sol-gel, solvothermal, and coprecipitation) for MFe2O4 were further compared. Characterization showed that sol-gel induced good purity, porous structure, large surface area, and favorable element chemical states for ferrite. Consequently, the as-synthesized CuFe2O4 showed better catalytic performance in the removal of organics (96.8% for Acid Orange 7 and 62.7% for diclofenac) along with good reusability compared with those obtained by solvothermal and coprecipitation routes. This work provides a deeper understanding of spinel ferrite MFe2O4 synthesis and persulfate activation.
Using Electrolytic Manganese Residue to prepare novel nanocomposite catalysts for efficient degradation of Azo Dyes in Fenton-like processes.[Pubmed:32220714]
Chemosphere. 2020 Aug;252:126487.
In this study, Electrolytic Manganese Residue (EMR) was treated by EDTA-2Na/NaOH, ultrasonic etching, and hydrothermal reaction to obtain a novel nanocomposite catalyst (called N-EMR), which then was used, together with H2O2, to treat synthetic textile wastewater containing Reactive Red X-3B, Methyl Orange, Methylene blue and Acid Orange 7. Results indicated that the N-EMR had a nano-sheet structure in sizes of 100-200 nm; new iron and manganese oxides with high activity were produced. The mixture of a small amount of N-EMR (40 mg/L) and H2O2 (0.4 x 10(-3) M) could removal about 99% of azo dyes (at 100 mg/L in 100 mL) within 6-15 min, much faster than many advanced oxidation processes (AOPs) reported in the literature. The elucidation of the associated mechanism for azo dyes degradation indicates that azo dyes were attacked by superoxide radicals, hydroxyl radicals, and electron holes generated within system. N-EMR was found to be reusable and showed limited inhibition by co-existing anions and cations. Moreover, high removal efficiency of azo dyes could happen in the system with a wide range of pH (1-8.5) and temperatures (25-45 degrees C), indicating that the process developed in this study may have broad application potential in treatment of azo dyes contaminated wastewater.
Different refractory organic substances degradation and microbial community shift in the single-chamber bio-photoelectrochemical system.[Pubmed:32203871]
Bioresour Technol. 2020 Jul;307:123176.
The single-chamber bio-photoelectrochemical system (BPES) with a BiOBr photocathode was developed for Acid Orange 7 (AO7), 2,4 dichlorophenol (2,4-DCP) and chloramphenicol (CAP) degradation under solar irradiation. Photoelectrochemical characterizations showed that the optimized BiOBr-photocathode exhibited great light-response property and excellent electrochemcial performance. Moreover, desired TOC removals were achieved for various organic pollutants, with the values of 90.97% (AO7), 81.41% (2,4-DCP) and 78.47% (CAP). Besides, the lower cathode potentials in the illuminated BPESs were favorable to efficient pollutants degradation. Significant microbial community shifts were observed among the inoculation and anodic biofilms from the BPES, and the most dominated species in anodic biofilms acclimated to various pollutants were Geobacter and Pseudomonas, which have the abilities of extracellular electrons transfer and organics degradation. Some other species that different from the inoculation were also identified from the BPES biofilms. This study suggested that BPES had great potential for refractory organics degradation.
Construction of TiO2/Ag3PO4 nanojunctions on carbon fiber cloth for photocatalytically removing various organic pollutants in static or flowing wastewater.[Pubmed:32200165]
J Colloid Interface Sci. 2020 Jul 1;571:213-221.
Plenty of power-shaped semiconductor nanomaterials have been used to photocatalytically degrade various pollutant wastewater in beakers, but they are difficult to be applied in the practical wastewater that is flowing in river or pipeline. Thus, the key to photocatalytically degrading the flowing wastewater is to develop flexible large-scale filter-membrane with high photocatalytic activity. To address the issue, with carbon fiber cloth (CFC) as the porous substrate and TiO2/Ag3PO4 as ultraviolet/visible (UV/Vis) responsed components, we reported the in-situ growth of TiO2/Ag3PO4 nanojunctions on CFC as filter-membrane-shaped photocatalyst. The resulting CFC/TiO2/Ag3PO4 is composed of CFC whose surface is decorated with TiO2 nanorods (length: 1 +/- 0.5 mum, diameter: 150 +/- 50 nm) and Ag3PO4 nanoparticles (diameter: 20-100 nm). CFC/TiO2/Ag3PO4 displays a broad absorption region with two edges (~410 and ~510 nm), owing to the bandgaps of TiO2 and Ag3PO4. Under Vis or UV-Vis light illumination, CFC/TiO2/Ag3PO4 (4 x 4 cm(2)) can efficiently degrade more phenol (80.6%/89.4%), tetracycline (TC, 91.7%/94.2%), rhodamine B (RhB, 98.4%/99.5%) and Acid Orange 7 (AO7, 97.6%/98.3%) in the beaker than CFC/TiO2 or CFC/Ag3PO4. Especially, CFC/TiO2/Ag3PO4 (diameter: ~10 cm) as the filter-membrane was used to construct multiple device for degrading the flowing RhB wastewater. The removal efficiency of RhB increases from 19.6% at the 1st pool to 96.8% at the 8th pool. Therefore, this study brings some insights for purifying organic pollutants in static or flowing wastewater by using filter-membrane-shaped photocatalysts.
Hierarchical MnO2 nanoflowers blooming on 3D nickel foam: A novel micro-macro catalyst for peroxymonosulfate activation.[Pubmed:32199267]
J Colloid Interface Sci. 2020 Jul 1;571:142-154.
In this work, birnessite-type delta-MnO2 nanoflowers were uniformly deposited on 3D nickel foam (NF) by one-step hydrothermal route for high-efficient activation of peroxymonosulfate (PMS) towards degradation of Acid Orange 7 (AO7). High specific surface area, large pore volume and 3D hierarchical structure promotes the mass and electron transfer for great catalytic activity. Low reaction energy barrier (Ea = 27.5 kJ/mol) and outstanding reusability with extremely low manganese leaching during recycling (<0.06 mg/L) was achieved due to the 3D hierarchical structure which could effectively avoid the agglomeration of nano-sized MnO2. SO4(-) was confirmed to be the predominant reactive species for AO7 decomposition by electron spin resonance and quenching tests. The synergistic catalytic mechanism of MnO2/NF and the role of inner-sphere complexation between the active sites of MnO2 and peroxymonosulfate were thoroughly investigated. Compared with traditional nano/micro-sized catalysts, 3D macroscopic MnO2/NF with facile recovery and high stability potentially facilitates fascinating applications as green heterogeneous catalysis approach.


